Lipid metabolic reprogramming in cancer cells
- PMID: 26807644
- PMCID: PMC4728678
- DOI: 10.1038/oncsis.2015.49
Lipid metabolic reprogramming in cancer cells
Abstract
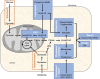
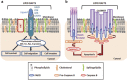
Many human diseases, including metabolic, immune and central nervous system disorders, as well as cancer, are the consequence of an alteration in lipid metabolic enzymes and their pathways. This illustrates the fundamental role played by lipids in maintaining membrane homeostasis and normal function in healthy cells. We reviewed the major lipid dysfunctions occurring during tumor development, as determined using systems biology approaches. In it, we provide detailed insight into the essential roles exerted by specific lipids in mediating intracellular oncogenic signaling, endoplasmic reticulum stress and bidirectional crosstalk between cells of the tumor microenvironment and cancer cells. Finally, we summarize the advances in ongoing research aimed at exploiting the dependency of cancer cells on lipids to abolish tumor progression.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

