Epidemiology of Acute Respiratory Distress Syndrome Following Hematopoietic Stem Cell Transplantation
- PMID: 26807683
- PMCID: PMC4868669
- DOI: 10.1097/CCM.0000000000001617
Epidemiology of Acute Respiratory Distress Syndrome Following Hematopoietic Stem Cell Transplantation
Abstract
Objectives: Pulmonary complications are common following hematopoietic stem cell transplantation. Numerous idiopathic post-transplantation pulmonary syndromes have been described. Patients at the severe end of this spectrum may present with hypoxemic respiratory failure and pulmonary infiltrates, meeting criteria for acute respiratory distress syndrome. The incidence and outcomes of acute respiratory distress syndrome in this setting are poorly characterized.
Design: Retrospective cohort study.
Setting: Mayo Clinic, Rochester, MN.
Patients: Patients undergoing autologous and allogeneic hematopoietic stem cell transplantation between January 1, 2005, and December 31, 2012.
Interventions: None.
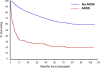
Measurements and main results: Patients were screened for acute respiratory distress syndrome development within 1 year of hematopoietic stem cell transplantation. Acute respiratory distress syndrome adjudication was performed in accordance with the 2012 Berlin criteria. In total, 133 cases of acute respiratory distress syndrome developed in 2,635 patients undergoing hematopoietic stem cell transplantation (5.0%). Acute respiratory distress syndrome developed in 75 patients (15.6%) undergoing allogeneic hematopoietic stem cell transplantation and 58 patients (2.7%) undergoing autologous hematopoietic stem cell transplantation. Median time to acute respiratory distress syndrome development was 55.4 days (interquartile range, 15.1-139 d) in allogeneic hematopoietic stem cell transplantation and 14.2 days (interquartile range, 10.5-124 d) in autologous hematopoietic stem cell transplantation. Twenty-eight-day mortality was 46.6%. At 12 months following hematopoietic stem cell transplantation, 89 patients (66.9%) who developed acute respiratory distress syndrome had died. Only 7 of 133 acute respiratory distress syndrome cases met criteria for engraftment syndrome and 15 for diffuse alveolar hemorrhage.
Conclusions: Acute respiratory distress syndrome is a frequent complication following hematopoietic stem cell transplantation, dramatically influencing patient-important outcomes. Most cases of acute respiratory distress syndrome following hematopoietic stem cell transplantation do not meet criteria for a more specific post-transplantation pulmonary syndrome. These findings highlight the need to better understand the risk factors underlying acute respiratory distress syndrome in this population, thereby facilitating the development of effective prevention strategies.
Conflict of interest statement
The remaining authors have disclosed that they do not have any potential conflicts of interest.
Figures


Comment in
-
Blood, Sweat, and teARDS.Crit Care Med. 2016 Jun;44(6):1235-6. doi: 10.1097/CCM.0000000000001689. Crit Care Med. 2016. PMID: 27182855 No abstract available.
References
-
- Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354(17):1813–1826. - PubMed
-
- Afessa B, Abdulai RM, Kremers WK, et al. Risk factors and outcome of pulmonary complications after autologous hematopoietic stem cell transplant. Chest. 2012;141(2):442–450. - PubMed
-
- Afessa B, Litzow MR, Tefferi A. Bronchiolitis obliterans and other late onset non-infectious pulmonary complications in hematopoietic stem cell transplantation. Bone marrow transplantation. 2001;28(5):425–434. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

