Protein Ensembles: How Does Nature Harness Thermodynamic Fluctuations for Life? The Diverse Functional Roles of Conformational Ensembles in the Cell
- PMID: 26807783
- PMCID: PMC6407618
- DOI: 10.1021/acs.chemrev.5b00562
Protein Ensembles: How Does Nature Harness Thermodynamic Fluctuations for Life? The Diverse Functional Roles of Conformational Ensembles in the Cell
Abstract
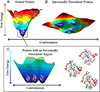
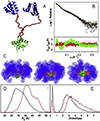
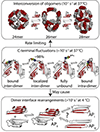
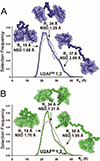
All soluble proteins populate conformational ensembles that together constitute the native state. Their fluctuations in water are intrinsic thermodynamic phenomena, and the distributions of the states on the energy landscape are determined by statistical thermodynamics; however, they are optimized to perform their biological functions. In this review we briefly describe advances in free energy landscape studies of protein conformational ensembles. Experimental (nuclear magnetic resonance, small-angle X-ray scattering, single-molecule spectroscopy, and cryo-electron microscopy) and computational (replica-exchange molecular dynamics, metadynamics, and Markov state models) approaches have made great progress in recent years. These address the challenging characterization of the highly flexible and heterogeneous protein ensembles. We focus on structural aspects of protein conformational distributions, from collective motions of single- and multi-domain proteins, intrinsically disordered proteins, to multiprotein complexes. Importantly, we highlight recent studies that illustrate functional adjustment of protein conformational ensembles in the crowded cellular environment. We center on the role of the ensemble in recognition of small- and macro-molecules (protein and RNA/DNA) and emphasize emerging concepts of protein dynamics in enzyme catalysis. Overall, protein ensembles link fundamental physicochemical principles and protein behavior and the cellular network and its regulation.
Figures






References
-
- Frauenfelder H; Sligar SG; Wolynes PG The energy landscapes and motions of proteins. Science 1991, 254, 1598–1603. - PubMed
-
- Nagaraju M; McGowan LC; Hamelberg D Cyclophilin A inhibition: targeting transition-state-bound enzyme conformations for structure-based drug design. J. Chem. Inf. Model 2013, 53, 403–410. - PubMed
-
- Karplus M; Weaver DL Protein-folding dynamics. Nature 1976, 260, 404–406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

