Real-Time Investigation of Tuberculosis Transmission: Developing the Respiratory Aerosol Sampling Chamber (RASC)
- PMID: 26807816
- PMCID: PMC4726558
- DOI: 10.1371/journal.pone.0146658
Real-Time Investigation of Tuberculosis Transmission: Developing the Respiratory Aerosol Sampling Chamber (RASC)
Abstract
Knowledge of the airborne nature of respiratory disease transmission owes much to the pioneering experiments of Wells and Riley over half a century ago. However, the mechanical, physiological, and immunopathological processes which drive the production of infectious aerosols by a diseased host remain poorly understood. Similarly, very little is known about the specific physiological, metabolic and morphological adaptations which enable pathogens such as Mycobacterium tuberculosis (Mtb) to exit the infected host, survive exposure to the external environment during airborne carriage, and adopt a form that is able to enter the respiratory tract of a new host, avoiding innate immune and physical defenses to establish a nascent infection. As a first step towards addressing these fundamental knowledge gaps which are central to any efforts to interrupt disease transmission, we developed and characterized a small personal clean room comprising an array of sampling devices which enable isolation and representative sampling of airborne particles and organic matter from tuberculosis (TB) patients. The complete unit, termed the Respiratory Aerosol Sampling Chamber (RASC), is instrumented to provide real-time information about the particulate output of a single patient, and to capture samples via a suite of particulate impingers, impactors and filters. Applying the RASC in a clinical setting, we demonstrate that a combination of molecular and microbiological assays, as well as imaging by fluorescence and scanning electron microscopy, can be applied to investigate the identity, viability, and morphology of isolated aerosolized particles. Importantly, from a preliminary panel of active TB patients, we observed the real-time production of large numbers of airborne particles including Mtb, as confirmed by microbiological culture and polymerase chain reaction (PCR) genotyping. Moreover, direct imaging of captured samples revealed the presence of multiple rod-like Mtb organisms whose physical dimensions suggested the capacity for travel deep into the alveolar spaces of the human lung.
Conflict of interest statement
Figures

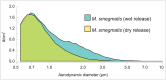



References
-
- Riley RL, Mills CC, Nyka W, Weinstock N, Storey PB, Sultan LU, et al. (1959) Aerial dissemination of pulmonary tuberculosis. A two-year study of contagion in a tuberculosis ward. Am J Hygiene 70: 2. - PubMed
-
- Riley RL, Mills CC, O'Grady F, Sultan LU, Wittstadt F, Shivpuri DN (1962) Infectiousness of air from a tuberculosis ward. Ultraviolet irradiation of infected air: comparative infectiousness of different patients. Am Rev Respir Dis 85: 511–525. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

