Comparative Study of Injury Models for Studying Muscle Regeneration in Mice
- PMID: 26807982
- PMCID: PMC4726569
- DOI: 10.1371/journal.pone.0147198
Comparative Study of Injury Models for Studying Muscle Regeneration in Mice
Abstract
Background: A longstanding goal in regenerative medicine is to reconstitute functional tissues or organs after injury or disease. Attention has focused on the identification and relative contribution of tissue specific stem cells to the regeneration process. Relatively little is known about how the physiological process is regulated by other tissue constituents. Numerous injury models are used to investigate tissue regeneration, however, these models are often poorly understood. Specifically, for skeletal muscle regeneration several models are reported in the literature, yet the relative impact on muscle physiology and the distinct cells types have not been extensively characterised.
Methods: We have used transgenic Tg:Pax7nGFP and Flk1GFP/+ mouse models to respectively count the number of muscle stem (satellite) cells (SC) and number/shape of vessels by confocal microscopy. We performed histological and immunostainings to assess the differences in the key regeneration steps. Infiltration of immune cells, chemokines and cytokines production was assessed in vivo by Luminex®.
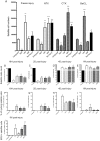
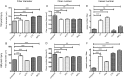
Results: We compared the 4 most commonly used injury models i.e. freeze injury (FI), barium chloride (BaCl2), notexin (NTX) and cardiotoxin (CTX). The FI was the most damaging. In this model, up to 96% of the SCs are destroyed with their surrounding environment (basal lamina and vasculature) leaving a "dead zone" devoid of viable cells. The regeneration process itself is fulfilled in all 4 models with virtually no fibrosis 28 days post-injury, except in the FI model. Inflammatory cells return to basal levels in the CTX, BaCl2 but still significantly high 1-month post-injury in the FI and NTX models. Interestingly the number of SC returned to normal only in the FI, 1-month post-injury, with SCs that are still cycling up to 3-months after the induction of the injury in the other models.
Conclusions: Our studies show that the nature of the injury model should be chosen carefully depending on the experimental design and desired outcome. Although in all models the muscle regenerates completely, the trajectories of the regenerative process vary considerably. Furthermore, we show that histological parameters are not wholly sufficient to declare that regeneration is complete as molecular alterations (e.g. cycling SCs, cytokines) could have a major persistent impact.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

