Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice
- PMID: 26808346
- PMCID: PMC4825868
- DOI: 10.1038/nm.4030
Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice
Erratum in
-
Corrigendum: Long-term glycemic control using polymer-encapsulated human stem cell-derived beta cells in immune-competent mice.Nat Med. 2016 Apr;22(4):446. doi: 10.1038/nm0416-446e. Nat Med. 2016. PMID: 27050590 No abstract available.
Abstract
The transplantation of glucose-responsive, insulin-producing cells offers the potential for restoring glycemic control in individuals with diabetes. Pancreas transplantation and the infusion of cadaveric islets are currently implemented clinically, but these approaches are limited by the adverse effects of immunosuppressive therapy over the lifetime of the recipient and the limited supply of donor tissue. The latter concern may be addressed by recently described glucose-responsive mature beta cells that are derived from human embryonic stem cells (referred to as SC-β cells), which may represent an unlimited source of human cells for pancreas replacement therapy. Strategies to address the immunosuppression concerns include immunoisolation of insulin-producing cells with porous biomaterials that function as an immune barrier. However, clinical implementation has been challenging because of host immune responses to the implant materials. Here we report the first long-term glycemic correction of a diabetic, immunocompetent animal model using human SC-β cells. SC-β cells were encapsulated with alginate derivatives capable of mitigating foreign-body responses in vivo and implanted into the intraperitoneal space of C57BL/6J mice treated with streptozotocin, which is an animal model for chemically induced type 1 diabetes. These implants induced glycemic correction without any immunosuppression until their removal at 174 d after implantation. Human C-peptide concentrations and in vivo glucose responsiveness demonstrated therapeutically relevant glycemic control. Implants retrieved after 174 d contained viable insulin-producing cells.
Figures

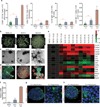
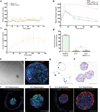
Comment in
-
Diabetes: Encapsulated β-cell implants enable glycaemic control.Nat Rev Endocrinol. 2016 Mar;12(3):126. doi: 10.1038/nrendo.2016.16. Epub 2016 Feb 5. Nat Rev Endocrinol. 2016. PMID: 26846347 No abstract available.
-
Immunoisolation of Human or Xenogeneic Insulin-Producing Cells: The Next Step for Treating Patients With Type 1 Diabetes?Transplantation. 2016 Aug;100(8):1592-4. doi: 10.1097/TP.0000000000001374. Transplantation. 2016. PMID: 27454914 No abstract available.
References
-
- Robertson RP. Islet transplantation as a treatment for diabetes - a work in progress. N Engl J Med. 2004;350:694–705. - PubMed
-
- Shapiro AM, et al. International trial of the Edmonton protocol for islet transplantation. N Engl J Med. 2006;355:1318–1330. - PubMed
-
- Hirshberg B. Lessons learned from the international trial of the edmonton protocol for islet transplantation. Current diabetes reports. 2007;7:301–303. - PubMed
-
- Lim F, Sun AM. Microencapsulated islets as bioartificial endocrine pancreas. Science. 1980;210:908–910. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- DE013023/DE/NIDCR NIH HHS/United States
- UC4 DK104218/DK/NIDDK NIH HHS/United States
- R01 EB000244/EB/NIBIB NIH HHS/United States
- U54 CA151884/CA/NCI NIH HHS/United States
- UC4DK104218/DK/NIDDK NIH HHS/United States
- P30 DK036836/DK/NIDDK NIH HHS/United States
- R01 DE013023/DE/NIDCR NIH HHS/United States
- EB000351/EB/NIBIB NIH HHS/United States
- Howard Hughes Medical Institute/United States
- CA151884/CA/NCI NIH HHS/United States
- R37 EB000244/EB/NIBIB NIH HHS/United States
- R01 EB000351/EB/NIBIB NIH HHS/United States
- EB000244/EB/NIBIB NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

