Acidic pH increases airway surface liquid viscosity in cystic fibrosis
- PMID: 26808501
- PMCID: PMC4767348
- DOI: 10.1172/JCI83922
Acidic pH increases airway surface liquid viscosity in cystic fibrosis
Abstract
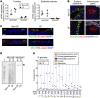
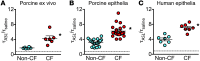
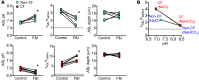
Cystic fibrosis (CF) disrupts respiratory host defenses, allowing bacterial infection, inflammation, and mucus accumulation to progressively destroy the lungs. Our previous studies revealed that mucus with abnormal behavior impaired mucociliary transport in newborn CF piglets prior to the onset of secondary manifestations. To further investigate mucus abnormalities, here we studied airway surface liquid (ASL) collected from newborn piglets and ASL on cultured airway epithelia. Fluorescence recovery after photobleaching revealed that the viscosity of CF ASL was increased relative to that of non-CF ASL. CF ASL had a reduced pH, which was necessary and sufficient for genotype-dependent viscosity differences. The increased viscosity of CF ASL was not explained by pH-independent changes in HCO3- concentration, altered glycosylation, additional pH-induced disulfide bond formation, increased percentage of nonvolatile material, or increased sulfation. Treating acidic ASL with hypertonic saline or heparin largely reversed the increased viscosity, suggesting that acidic pH influences mucin electrostatic interactions. These findings link loss of cystic fibrosis transmembrane conductance regulator-dependent alkalinization to abnormal CF ASL. In addition, we found that increasing Ca2+ concentrations elevated ASL viscosity, in part, independently of pH. The results suggest that increasing pH, reducing Ca2+ concentration, and/or altering electrostatic interactions in ASL might benefit early CF.
Figures
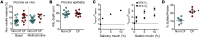


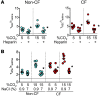

References
-
- Quinton P. Physiological basis of cystic fibrosis: a historical perspective. Physiol Rev. 1999;79(suppl 1):S3–S22. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- P01 HL107150/HL/NHLBI NIH HHS/United States
- P01 HL091842/HL/NHLBI NIH HHS/United States
- NIH P41GM10349010/GM/NIGMS NIH HHS/United States
- P01 HL051670/HL/NHLBI NIH HHS/United States
- P30 ES005605/ES/NIEHS NIH HHS/United States
- T35 HL007485/HL/NHLBI NIH HHS/United States
- R01 GM032373/GM/NIGMS NIH HHS/United States
- R01GM32373/GM/NIGMS NIH HHS/United States
- P41 GM103490/GM/NIGMS NIH HHS/United States
- HL091842/HL/NHLBI NIH HHS/United States
- HL11744/HL/NHLBI NIH HHS/United States
- HL51670/HL/NHLBI NIH HHS/United States
- Howard Hughes Medical Institute/United States
- T32 GM007337/GM/NIGMS NIH HHS/United States
- DP2 HL117744/HL/NHLBI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

