Effects of Obstacles on the Dynamics of Kinesins, Including Velocity and Run Length, Predicted by a Model of Two Dimensional Motion
- PMID: 26808534
- PMCID: PMC4726810
- DOI: 10.1371/journal.pone.0147676
Effects of Obstacles on the Dynamics of Kinesins, Including Velocity and Run Length, Predicted by a Model of Two Dimensional Motion
Abstract
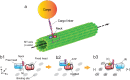
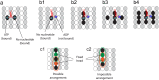
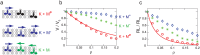
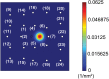
Kinesins are molecular motors which walk along microtubules by moving their heads to different binding sites. The motion of kinesin is realized by a conformational change in the structure of the kinesin molecule and by a diffusion of one of its two heads. In this study, a novel model is developed to account for the 2D diffusion of kinesin heads to several neighboring binding sites (near the surface of microtubules). To determine the direction of the next step of a kinesin molecule, this model considers the extension in the neck linkers of kinesin and the dynamic behavior of the coiled-coil structure of the kinesin neck. Also, the mechanical interference between kinesins and obstacles anchored on the microtubules is characterized. The model predicts that both the kinesin velocity and run length (i.e., the walking distance before detaching from the microtubule) are reduced by static obstacles. The run length is decreased more significantly by static obstacles than the velocity. Moreover, our model is able to predict the motion of kinesin when other (several) motors also move along the same microtubule. Furthermore, it suggests that the effect of mechanical interaction/interference between motors is much weaker than the effect of static obstacles. Our newly developed model can be used to address unanswered questions regarding degraded transport caused by the presence of excessive tau proteins on microtubules.
Conflict of interest statement
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

