Replication-Competent Influenza A and B Viruses Expressing a Fluorescent Dynamic Timer Protein for In Vitro and In Vivo Studies
- PMID: 26809059
- PMCID: PMC4725730
- DOI: 10.1371/journal.pone.0147723
Replication-Competent Influenza A and B Viruses Expressing a Fluorescent Dynamic Timer Protein for In Vitro and In Vivo Studies
Abstract
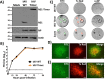
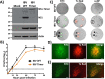
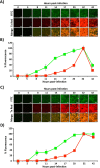
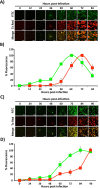
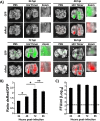
Influenza A and B viruses (IAV and IBV, respectively) cause annual seasonal human respiratory disease epidemics. In addition, IAVs have been implicated in occasional pandemics with inordinate health and economic consequences. Studying influenza viruses in vitro or in vivo requires the use of laborious secondary methodologies to identify infected cells. To circumvent this requirement, replication-competent infectious influenza viruses expressing an easily traceable fluorescent reporter protein can be used. Timer is a fluorescent protein that undergoes a time-dependent color emission conversion from green to red. The rate of spectral change is independent of Timer protein concentration and can be used to chronologically measure the duration of its expression. Here, we describe the generation of replication-competent IAV and IBV where the viral non-structural protein 1 (NS1) was fused to the fluorescent dynamic Timer protein. Timer-expressing IAV and IBV displayed similar plaque phenotypes and growth kinetics to wild-type viruses in tissue culture. Within infected cells, Timer's spectral shift can be used to measure the rate and cell-to-cell spread of infection using fluorescent microscopy, plate readers, or flow cytometry. The progression of Timer-expressing IAV infection was also evaluated in a mouse model, demonstrating the feasibility to characterize IAV cell-to-cell infections in vivo. By providing the ability to chronologically track viral spread, Timer-expressing influenza viruses are an excellent option to evaluate the in vitro and in vivo dynamics of viral infection.
Conflict of interest statement
Figures

References
-
- CDC. Deaths: Final Data for 2013. 2013 February 6. Report No.
-
- WHO. Influenza (Seasonal) Fact Sheet N211 http://www.who.int/mediacentre/factsheets/fs211/en/2014 [November 2]. Available from: http://www.who.int/mediacentre/factsheets/fs211/en/.
-
- CDC. Updated CDC Estimates of 2009 H1N1 Influenza Cases, Hospitalizations and Deaths in the United States, April 2009–April 10, 2010 2010 [updated May 14; cited 2013]. Available from: http://www.cdc.gov/h1n1flu/estimates_2009_h1n1.htm.
-
- Dawood FS, Iuliano AD, Reed C, Meltzer MI, Shay DK, Cheng P-Y, et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: a modelling study. The Lancet Infectious Diseases. 2012;12(9):687–95. 10.1016/S1473-3099(12)70121-4 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

