A Fluorescent Oligothiophene-Bis-Triazine ligand interacts with PrP fibrils and detects SDS-resistant oligomers in human prion diseases
- PMID: 26809712
- PMCID: PMC4727337
- DOI: 10.1186/s13024-016-0074-7
A Fluorescent Oligothiophene-Bis-Triazine ligand interacts with PrP fibrils and detects SDS-resistant oligomers in human prion diseases
Abstract
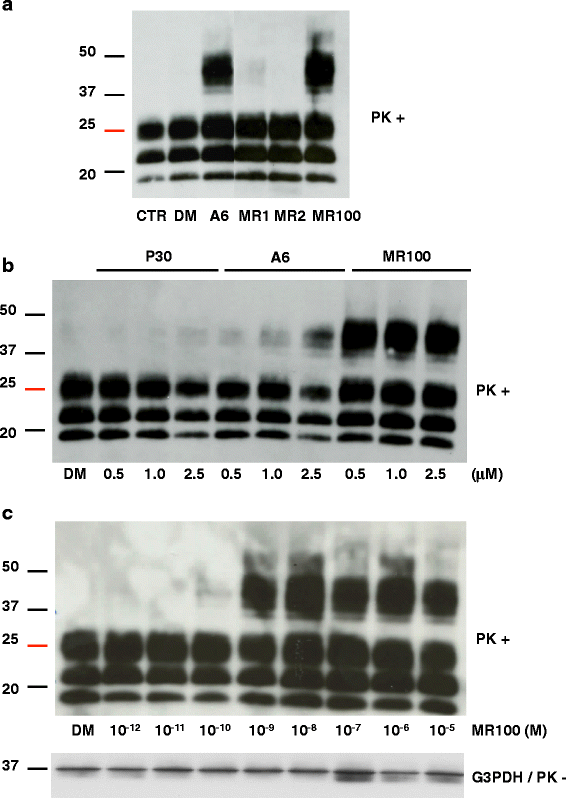
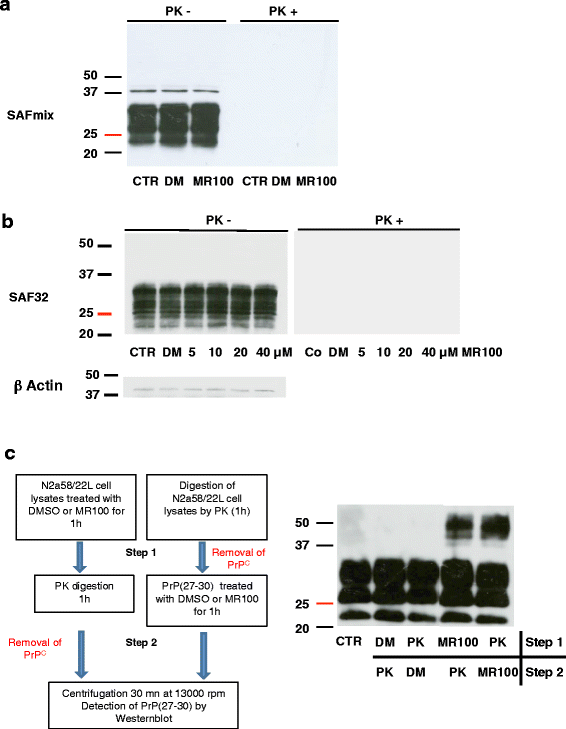
Background: Prion diseases are characterized by the accumulation in the central nervous system of an abnormally folded isoform of the prion protein, named PrP(Sc). Aggregation of PrP(Sc) into oligomers and fibrils is critically involved in the pathogenesis of prion diseases. Oligomers are supposed to be the key neurotoxic agents in prion disease, so modulation of prion aggregation pathways with small molecules can be a valuable strategy for studying prion pathogenicity and for developing new diagnostic and therapeutic approaches. We previously identified thienyl pyrimidine compounds that induce SDS-resistant PrP(Sc) (rSDS-PrP(Sc)) oligomers in prion-infected samples.
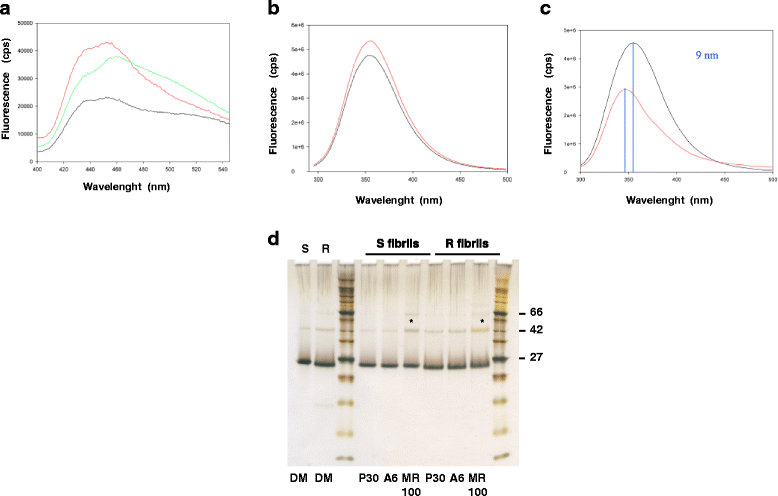
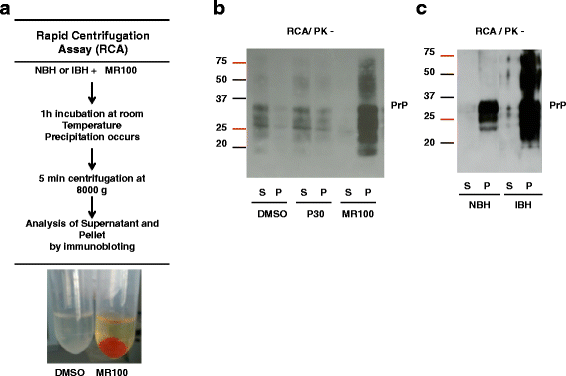
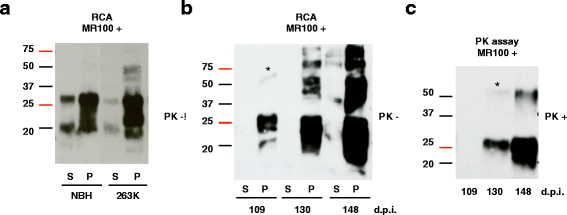
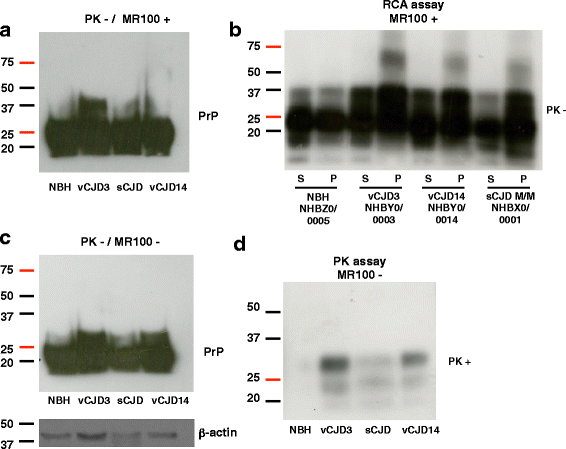
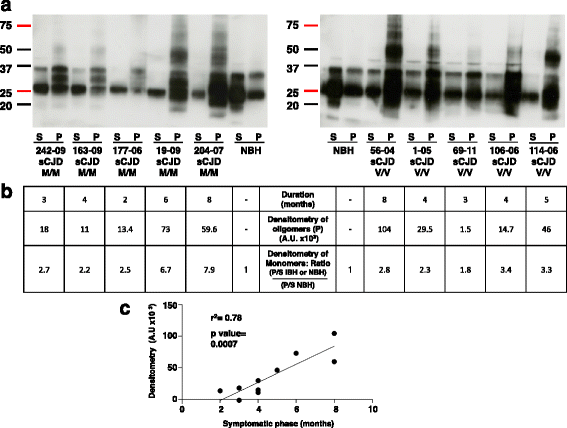
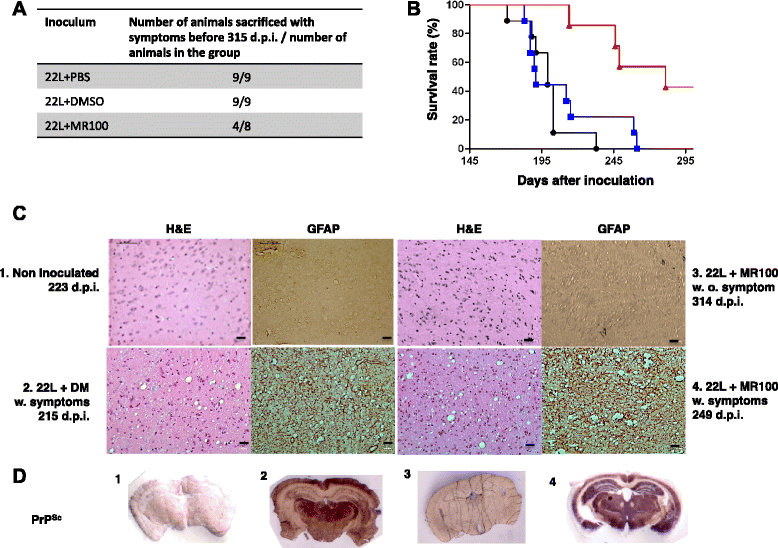
Results: Due to the low effective doses of the thienyl pyrimidine hits, we synthesized a quaterthiophene-bis-triazine compound, called MR100 to better evaluate their diagnostic and therapeutic potentials. This molecule exhibits a powerful activity inducing rSDS-PrP(Sc) oligomers at nanomolar concentrations in prion-infected cells. Fluorescence interaction studies of MR100 with mouse PrP fibrils showed substantial modification of the spectrum, and the interaction was confirmed in vitro by production of rSDS-oligomer species upon incubation of MR100 with fibrils in SDS-PAGE gel. We further explored whether MR100 compound has a potential to be used in the diagnosis of prion diseases. Our results showed that: (i) MR100 can detect rSDS-oligomers in prion-infected brain homogenates of various species, including human samples from CJD patients; (ii) A protocol, called "Rapid Centrifugation Assay" (RCA), was developed based on MR100 property of inducing rSDS-PrP(Sc) oligomers only in prion-infected samples, and avoiding the protease digestion step. RCA allows the detection of both PK-sensitive and PK-resistant PrP(Sc) species in rodents samples but also from patients with different CJD forms (sporadic and new variant); (iii) A correlation could be established between the amount of rSDS-PrP(Sc) oligomers revealed by MR100 and the duration of the symptomatic phase of the disease in CJD patients; and (iv) Bioassay experiments showed that MR100 can trap prion infectivity more efficiently than P30 drug.
Conclusions: MR100 is a powerful tool not only for studying the prion aggregation pathways regarding oligomeric and sPrP(Sc) species, but also for developing alternative methods for the detection of prion-infected samples. Considering our bioassay results, MR100 is a promising molecule for the development of prion decontamination approaches.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

