The development and validation of the Leiden Bother and Needs Questionnaire for patients with pituitary disease: the LBNQ-Pituitary
- PMID: 26809957
- PMCID: PMC4858557
- DOI: 10.1007/s11102-016-0707-4
The development and validation of the Leiden Bother and Needs Questionnaire for patients with pituitary disease: the LBNQ-Pituitary
Abstract
Background: Patients report persisting impairment in quality of life (QoL) after treatment for pituitary disease. At present, there is no questionnaire to assess (a) whether patients with pituitary disease are bothered by these consequences, and (b) their needs for support.
Objective: To develop and validate a disease-specific questionnaire for patients with pituitary disease which incorporates patient perceived bother related to the consequences of the disease, and their needs for support.
Methods: Items for the Leiden Bother and Needs Questionnaire for patients with pituitary disease (LBNQ-Pituitary) were formulated based on results of a recent focus group study (n = 49 items). 337 patients completed the LBNQ-Pituitary and six validated QoL questionnaires (EuroQoL-5D, SF-36, MFI-20, HADS, AcroQol, CushingQoL). Construct validity was examined by exploratory factor analysis. Reliabilities of the subscales were calculated with Cronbach's alphas, and concurrent validity was assessed by calculating Spearman's correlations between the LBNQ-Pituitary and the other measures.
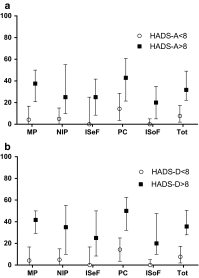
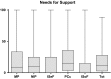
Results: Factor analyses produced five subscales (i.e., mood problems, negative illness perceptions, issues in sexual functioning, physical and cognitive complaints, issues in social functioning) containing a total of 26 items. All factors were found to be reliable (Cronbach's alphas all ≥.765), and the correlations between the dimensions of the LBNQ-Pituitary and other questionnaires (all P ≤ .0001) demonstrated convergent validity.
Conclusions: The LBNQ-Pituitary can be used to assess the degree to which patients are bothered by the consequences of the pituitary disease, as well as their needs for support. It could also facilitate an efficient assessment of patients' needs for support in clinical practice. We postulate that paying attention to needs for support will lead to optimal patient care (e.g., improvement in psychosocial care), and positively affect QoL.
Keywords: Acromegaly; Cushing’s disease; Cushing’s syndrome; Hypopituitarism; Illness perceptions; Needs; Non-functioning pituitary macroadenoma; Patient-reported-outcome; Pituitary adenomas; Prolactinoma; Quality of life.
Figures
Similar articles
-
Development and validation of the disease-specific QOL-CD quality of life questionnaire for patients with Cushing's disease.Neurosurg Focus. 2020 Jun;48(6):E4. doi: 10.3171/2020.3.FOCUS2044. Neurosurg Focus. 2020. PMID: 32480368
-
Quality of life in patients with a pituitary adenoma.Pituitary. 2003 Sep;6(2):81-7. doi: 10.1023/b:pitu.0000004798.27230.ed. Pituitary. 2003. PMID: 14703017 Clinical Trial.
-
Towards a better quality of life (QoL) for patients with pituitary diseases: results from a focus group study exploring QoL.Pituitary. 2015 Feb;18(1):86-100. doi: 10.1007/s11102-014-0561-1. Pituitary. 2015. PMID: 24682940
-
[New developments in pituitary diseases].Dtsch Med Wochenschr. 2012 Dec;137(49):2540-2. doi: 10.1055/s-0032-1327285. Epub 2012 Nov 27. Dtsch Med Wochenschr. 2012. PMID: 23188633 Review. German. No abstract available.
-
Quality of life in Cushing's syndrome.Pituitary. 2015 Apr;18(2):195-200. doi: 10.1007/s11102-015-0640-y. Pituitary. 2015. PMID: 25647329 Review.
Cited by
-
Quality of life in Prolactinoma: A systematic review.Pituitary. 2024 Jun;27(3):239-247. doi: 10.1007/s11102-024-01392-1. Epub 2024 Apr 24. Pituitary. 2024. PMID: 38656635 Free PMC article.
-
Quality of life in non-functioning pituitary adenoma: A systematic review.Neurosurg Rev. 2024 Nov 23;47(1):867. doi: 10.1007/s10143-024-03126-0. Neurosurg Rev. 2024. PMID: 39578273
-
Support Needs of Patients with Cushing's Disease and Cushing's Syndrome: Results of a Survey Conducted in Germany and the USA.Int J Endocrinol. 2018 Oct 9;2018:9014768. doi: 10.1155/2018/9014768. eCollection 2018. Int J Endocrinol. 2018. PMID: 30402098 Free PMC article.
-
A patient-reported outcome measure for patients with pituitary adenoma undergoing transsphenoidal surgery.Pituitary. 2022 Aug;25(4):673-683. doi: 10.1007/s11102-022-01251-x. Epub 2022 Jul 15. Pituitary. 2022. PMID: 35838913 Free PMC article.
-
How non-functioning pituitary adenomas can affect health-related quality of life: a conceptual model and literature review.Pituitary. 2018 Apr;21(2):208-216. doi: 10.1007/s11102-017-0860-4. Pituitary. 2018. PMID: 29302835 Free PMC article. Review.
References
-
- Gardner D, Shoback D. Basic and clinical endocrinology. 9. Singapore: McGrawHill; 2011. pp. 100–101.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

