Uncovering an Important Role for YopJ in the Inhibition of Caspase-1 in Activated Macrophages and Promoting Yersinia pseudotuberculosis Virulence
- PMID: 26810037
- PMCID: PMC4807483
- DOI: 10.1128/IAI.00843-15
Uncovering an Important Role for YopJ in the Inhibition of Caspase-1 in Activated Macrophages and Promoting Yersinia pseudotuberculosis Virulence
Abstract
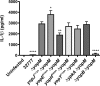
Pathogenic Yersinia species utilize a type III secretion system to translocate Yop effectors into infected host cells. Yop effectors inhibit innate immune responses in infected macrophages to promote Yersinia pathogenesis. In turn,Yersinia-infected macrophages respond to translocation of Yops by activating caspase-1, but different mechanisms of caspase-1 activation occur, depending on the bacterial genotype and the state of phagocyte activation. In macrophages activated with lipopolysaccharide (LPS) prior to Yersinia pseudotuberculosis infection, caspase-1 is activated by a rapid inflammasome-dependent mechanism that is inhibited by translocated YopM. The possibility that other effectors cooperate with YopM to inhibit caspase-1 activation in LPS-activated macrophages has not been investigated. Toward this aim, epistasis analysis was carried out in which the phenotype of aY. pseudotuberculosis yopM mutant was compared to that of a yopJ yopM, yopE yopM, yopH yopM, yopT yopM, or ypkA yopM mutant. Activation of caspase-1 was measured by cleavage of the enzyme, release of interleukin-1β (IL-1β), and pyroptosis in LPS-activated macrophages infected with wild-type or mutant Y. pseudotuberculosis strains. Results show enhanced activation of caspase-1 after infection with the yopJ yopM mutant relative to infection by any other single or double mutant. Similar results were obtained with the yopJ, yopM, and yopJ yopM mutants ofY ersinia pestis Following intravenous infection of mice, theY. pseudotuberculosis yopJ mutant was as virulent as the wild type, while the yopJ yopM mutant was significantly more attenuated than the yopM mutant. In summary, through epistasis analysis this work uncovered an important role for YopJ in inhibiting caspase-1 in activated macrophages and in promoting Yersinia virulence.
Copyright © 2016, American Society for Microbiology. All Rights Reserved.
Figures
Similar articles
-
Manipulation of Interleukin-1β and Interleukin-18 Production by Yersinia pestis Effectors YopJ and YopM and Redundant Impact on Virulence.J Biol Chem. 2016 May 6;291(19):9894-905. doi: 10.1074/jbc.M115.697698. Epub 2016 Feb 16. J Biol Chem. 2016. PMID: 26884330 Free PMC article.
-
YopJ-induced caspase-1 activation in Yersinia-infected macrophages: independent of apoptosis, linked to necrosis, dispensable for innate host defense.PLoS One. 2012;7(4):e36019. doi: 10.1371/journal.pone.0036019. Epub 2012 Apr 26. PLoS One. 2012. PMID: 22563435 Free PMC article.
-
A Yersinia effector with enhanced inhibitory activity on the NF-κB pathway activates the NLRP3/ASC/caspase-1 inflammasome in macrophages.PLoS Pathog. 2011 Apr;7(4):e1002026. doi: 10.1371/journal.ppat.1002026. Epub 2011 Apr 21. PLoS Pathog. 2011. PMID: 21533069 Free PMC article.
-
Yersinia outer proteins: role in modulation of host cell signaling responses and pathogenesis.Annu Rev Microbiol. 2005;59:69-89. doi: 10.1146/annurev.micro.59.030804.121320. Annu Rev Microbiol. 2005. PMID: 15847602 Review.
-
The virulence plasmid of Yersinia, an antihost genome.Microbiol Mol Biol Rev. 1998 Dec;62(4):1315-52. doi: 10.1128/MMBR.62.4.1315-1352.1998. Microbiol Mol Biol Rev. 1998. PMID: 9841674 Free PMC article. Review.
Cited by
-
Role of the Yersinia pseudotuberculosis Virulence Plasmid in Pathogen-Phagocyte Interactions in Mesenteric Lymph Nodes.EcoSal Plus. 2021 Dec 15;9(2):eESP00142021. doi: 10.1128/ecosalplus.ESP-0014-2021. Epub 2021 Oct 27. EcoSal Plus. 2021. PMID: 34910573 Free PMC article. Review.
-
Pathogenicity and virulence of Yersinia.Virulence. 2024 Dec;15(1):2316439. doi: 10.1080/21505594.2024.2316439. Epub 2024 Feb 22. Virulence. 2024. PMID: 38389313 Free PMC article. Review.
-
The pyrin inflammasome and the Yersinia effector interaction.Immunol Rev. 2020 Sep;297(1):96-107. doi: 10.1111/imr.12907. Epub 2020 Jul 28. Immunol Rev. 2020. PMID: 32721043 Free PMC article. Review.
-
Methods for Detection of Pyrin Inflammasome Assembly in Macrophages Infected with Yersinia spp.Methods Mol Biol. 2019;2010:241-255. doi: 10.1007/978-1-4939-9541-7_17. Methods Mol Biol. 2019. PMID: 31177443 Free PMC article.
-
A motive for killing: effector functions of regulated lytic cell death.Immunol Cell Biol. 2017 Feb;95(2):146-151. doi: 10.1038/icb.2016.113. Epub 2016 Nov 9. Immunol Cell Biol. 2017. PMID: 27826146 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

