Yeast-Derived Particulate β-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer
- PMID: 26810222
- PMCID: PMC4761495
- DOI: 10.4049/jimmunol.1501853
Yeast-Derived Particulate β-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer
Erratum in
-
Correction: Yeast-Derived Particulate β-Glucan Treatment Subverts the Suppression of Myeloid-Derived Suppressor Cells (MDSC) by Inducing Polymorphonuclear MDSC Apoptosis and Monocytic MDSC Differentiation to APC in Cancer.J Immunol. 2016 May 1;196(9):3967. doi: 10.4049/jimmunol.1600346. J Immunol. 2016. PMID: 27183652 No abstract available.
Abstract
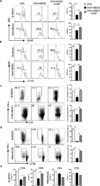
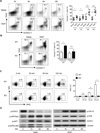
Myeloid-derived suppressor cells (MDSC) are a heterogeneous population of immature myeloid cells that promote tumor progression. In this study, we demonstrated that activation of a C-type lectin receptor, dectin-1, in MDSC differentially modulates the function of different MDSC subsets. Yeast-derived whole β-glucan particles (WGP; a ligand to engage and activate dectin-1, oral treatment in vivo) significantly decreased tumor weight and splenomegaly in tumor-bearing mice with reduced accumulation of polymorphonuclear MDSC but not monocytic MDSC (M-MDSC), and decreased polymorphonuclear MDSC suppression in vitro through the induction of respiratory burst and apoptosis. On a different axis, WGP-treated M-MDSC differentiated into F4/80(+)CD11c(+) cells in vitro that served as potent APC to induce Ag-specific CD4(+) and CD8(+) T cell responses in a dectin-1-dependent manner. Additionally, Erk1/2 phosphorylation was required for the acquisition of APC properties in M-MDSC. Moreover, WGP-treated M-MDSC differentiated into CD11c(+) cells in vivo with high MHC class II expression and induced decreased tumor burden when inoculated s.c. with Lewis lung carcinoma cells. This effect was dependent on the dectin-1 receptor. Strikingly, patients with non-small cell lung carcinoma that had received WGP treatment for 10-14 d prior to any other treatment had a decreased frequency of CD14(-)HLA-DR(-)CD11b(+)CD33(+) MDSC in the peripheral blood. Overall, these data indicate that WGP may be a potent immune modulator of MDSC suppressive function and differentiation in cancer.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures






Similar articles
-
β-Glucan enhances antitumor immune responses by regulating differentiation and function of monocytic myeloid-derived suppressor cells.Eur J Immunol. 2013 May;43(5):1220-30. doi: 10.1002/eji.201242841. Epub 2013 Mar 25. Eur J Immunol. 2013. PMID: 23424024
-
A circulating subpopulation of monocytic myeloid-derived suppressor cells as an independent prognostic/predictive factor in untreated non-small lung cancer patients.J Immunol Res. 2014;2014:659294. doi: 10.1155/2014/659294. Epub 2014 Nov 11. J Immunol Res. 2014. PMID: 25436215 Free PMC article.
-
Tumor-induced myeloid-derived suppressor cell subsets exert either inhibitory or stimulatory effects on distinct CD8+ T-cell activation events.Eur J Immunol. 2013 Nov;43(11):2930-42. doi: 10.1002/eji.201343349. Epub 2013 Aug 25. Eur J Immunol. 2013. PMID: 23878002
-
Myeloid-derived suppressors cells (MDSC) correlate with clinicopathologic factors and pathologic complete response (pCR) in patients with urothelial carcinoma (UC) undergoing cystectomy.Urol Oncol. 2018 Sep;36(9):405-412. doi: 10.1016/j.urolonc.2018.02.018. Epub 2018 Mar 30. Urol Oncol. 2018. PMID: 29606341 Review.
-
Plasticity of myeloid-derived suppressor cells in cancer.Curr Opin Immunol. 2018 Apr;51:76-82. doi: 10.1016/j.coi.2018.03.009. Epub 2018 Mar 14. Curr Opin Immunol. 2018. PMID: 29547768 Free PMC article. Review.
Cited by
-
Bio-Nanocarriers for Lung Cancer Management: Befriending the Barriers.Nanomicro Lett. 2021 Jun 12;13(1):142. doi: 10.1007/s40820-021-00630-6. Nanomicro Lett. 2021. PMID: 34138386 Free PMC article. Review.
-
Beta-glucan-induced inflammatory monocytes mediate antitumor efficacy in the murine lung.Cancer Immunol Immunother. 2018 Nov;67(11):1731-1742. doi: 10.1007/s00262-018-2234-9. Epub 2018 Aug 23. Cancer Immunol Immunother. 2018. PMID: 30167860 Free PMC article.
-
Yeast-Derived β-Glucan in Cancer: Novel Uses of a Traditional Therapeutic.Int J Mol Sci. 2019 Jul 24;20(15):3618. doi: 10.3390/ijms20153618. Int J Mol Sci. 2019. PMID: 31344853 Free PMC article. Review.
-
Mechanism-Driven and Clinically Focused Development of Botanical Foods as Multitarget Anticancer Medicine: Collective Perspectives and Insights from Preclinical Studies, IND Applications and Early-Phase Clinical Trials.Cancers (Basel). 2023 Jan 23;15(3):701. doi: 10.3390/cancers15030701. Cancers (Basel). 2023. PMID: 36765659 Free PMC article. Review.
-
Targeted deletion of PD-1 in myeloid cells induces antitumor immunity.Sci Immunol. 2020 Jan 3;5(43):eaay1863. doi: 10.1126/sciimmunol.aay1863. Sci Immunol. 2020. PMID: 31901074 Free PMC article.
References
-
- Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3:991–998. - PubMed
-
- Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–360. - PubMed
-
- Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science. 2011;331:1565–1570. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

