Extensive CD4 and CD8 T Cell Cross-Reactivity between Alphaherpesviruses
- PMID: 26810224
- PMCID: PMC4761520
- DOI: 10.4049/jimmunol.1502366
Extensive CD4 and CD8 T Cell Cross-Reactivity between Alphaherpesviruses
Abstract
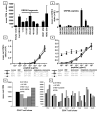
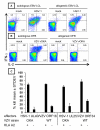
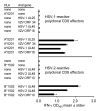
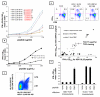
The Alphaherpesvirinae subfamily includes HSV types 1 and 2 and the sequence-divergent pathogen varicella zoster virus (VZV). T cells, controlled by TCR and HLA molecules that tolerate limited epitope amino acid variation, might cross-react between these microbes. We show that memory PBMC expansion with either HSV or VZV enriches for CD4 T cell lines that recognize the other agent at the whole-virus, protein, and peptide levels, consistent with bidirectional cross-reactivity. HSV-specific CD4 T cells recovered from HSV-seronegative persons can be explained, in part, by such VZV cross-reactivity. HSV-1-reactive CD8 T cells also cross-react with VZV-infected cells, full-length VZV proteins, and VZV peptides, as well as kill VZV-infected dermal fibroblasts. Mono- and cross-reactive CD8 T cells use distinct TCRB CDR3 sequences. Cross-reactivity to VZV is reconstituted by cloning and expressing TCRA/TCRB receptors from T cells that are initially isolated using HSV reagents. Overall, we define 13 novel CD4 and CD8 HSV-VZV cross-reactive epitopes and strongly imply additional cross-reactive peptide sets. Viral proteins can harbor both CD4 and CD8 HSV/VZV cross-reactive epitopes. Quantitative estimates of HSV/VZV cross-reactivity for both CD4 and CD8 T cells vary from 10 to 50%. Based on these findings, we hypothesize that host herpesvirus immune history may influence the pathogenesis and clinical outcome of subsequent infections or vaccinations for related pathogens and that cross-reactive epitopes and TCRs may be useful for multi-alphaherpesvirus vaccine design and adoptive cellular therapy.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures


References
-
- Baxter R, Tran TN, Ray P, Lewis E, Fireman B, Black S, Shinefield HR, Coplan PM, Saddier P. Impact of vaccination on the epidemiology of varicella: 1995-2009. Pediatrics. 2014;134:24–30. - PubMed
-
- Leung J, Harpaz R, Molinari NA, Jumaan A, Zhou F. Herpes zoster incidence among insured persons in the United States, 1993-2006: evaluation of impact of varicella vaccination. Clin. Infect. Dis. 2011;52:332–340. - PubMed
-
- Nysse LJ, Pinsky NA, Bratberg JP, Babar-Weber AY, Samuel TT, Krych EH, Ziegler AW, Jimale MA, Vierkant RA, Jacobson RM, Poland GA. Seroprevalence of antibody to varicella among Somali refugees. Mayo Clin. Proc. 2007;82:175–180. - PubMed
-
- Thomas F, Elguero E, Brodeur J, Le Goff J, Misse D. Herpes simplex virus type 2 and cancer: a medical geography approach. Infect. Genet. Evol. 2011;11:1239–1242. - PubMed
-
- Bradley H, Markowitz LE, Gibson T, McQuillan GM. Seroprevalence of herpes simplex virus types 1 and 2--United States, 1999-2010. J. Infect. Dis. 2014;209:325–333. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

