Disclosing Pleiotropic Effects During Genetic Risk Assessment for Alzheimer Disease: A Randomized Trial
- PMID: 26810768
- PMCID: PMC4979546
- DOI: 10.7326/M15-0187
Disclosing Pleiotropic Effects During Genetic Risk Assessment for Alzheimer Disease: A Randomized Trial
Abstract
Background: Increasing use of genetic testing raises questions about disclosing secondary findings, including pleiotropic information.
Objective: To determine the safety and behavioral effect of disclosing modest associations between apolipoprotein E (APOE) genotype and coronary artery disease (CAD) risk during APOE-based genetic risk assessments for Alzheimer disease (AD).
Design: Randomized, multicenter equivalence clinical trial. (ClinicalTrials.gov: NCT00462917).
Setting: 4 teaching hospitals.
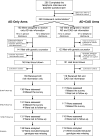
Participants: 257 asymptomatic adults were enrolled, 69% of whom had 1 AD-affected first-degree relative.
Intervention: Disclosure of genetic risk information about AD and CAD (AD+CAD) or AD only (AD-only).
Measurements: Primary outcomes were Beck Anxiety Inventory (BAI) and Center for Epidemiologic Studies Depression Scale (CES-D) scores at 12 months. Secondary outcomes were all measures at 6 weeks and 6 months and test-related distress and health behavior changes at 12 months.
Results: At 12 months, mean BAI scores were 3.5 in both the AD-only and AD+CAD groups (difference, 0.0 [95% CI, -1.0 to 1.0]), and mean CES-D scores were 6.4 and 7.1 in the AD-only and AD+CAD groups, respectively (difference, 0.7 [CI, -1.0 to 2.4]). Both confidence bounds fell within the equivalence margin of ±5 points. Among carriers of the APOE ε4 allele, distress was lower in the AD+CAD groups (difference, -4.8 [CI, -8.6 to -1.0]) (P = 0.031 for the interaction between group and APOE genotype). Participants in the AD+CAD groups also reported more health behavior changes, regardless of APOE genotype.
Limitations: Outcomes were self-reported by volunteers without severe anxiety, severe depression, or cognitive problems. Analyses omitted 33 randomly assigned participants.
Conclusion: Disclosure of pleiotropic information did not increase anxiety or depression and may have decreased distress among persons at increased risk for 2 conditions. Providing risk modification information about CAD improved health behaviors. Findings highlight the potential benefits of disclosure of secondary genetic findings when options exist for decreasing risk.
Primary funding source: National Human Genome Research Institute.
Figures

Comment in
-
Genomics: Prediction, Prevention, Priorities, and Punnett.Ann Intern Med. 2016 Feb 2;164(3):197-8. doi: 10.7326/M15-2993. Epub 2016 Jan 26. Ann Intern Med. 2016. PMID: 26810850 No abstract available.
-
Disclosing Pleiotropic Effects During Genetic Risk Assessment for Alzheimer Disease.Ann Intern Med. 2016 Nov 1;165(9):673. doi: 10.7326/L16-0400. Ann Intern Med. 2016. PMID: 27802468 No abstract available.
References
-
- Green RC, Rehm HL, Kohane IS. Clinical genome sequencing. In: Ginsberg GS, Willard HF, editors. Genomic and Personalized Medicine. 2. San Diego: Academic Press; 2013. pp. 102–22.
-
- Biesecker LG, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014;370:2418–25. - PubMed
-
- Korf BR. Integration of genomics into medical practice. Discov Med. 2013;16:241–8. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- AG013846/AG/NIA NIH HHS/United States
- F32 HG006993/HG/NHGRI NIH HHS/United States
- M01 RR000533/RR/NCRR NIH HHS/United States
- HG002213/HG/NHGRI NIH HHS/United States
- P30 AG013846/AG/NIA NIH HHS/United States
- UL1 TR001409/TR/NCATS NIH HHS/United States
- TR001102/TR/NCATS NIH HHS/United States
- U19 HD077671/HD/NICHD NIH HHS/United States
- HD077671/HD/NICHD NIH HHS/United States
- HG006500/HG/NHGRI NIH HHS/United States
- U01 HG006500/HG/NHGRI NIH HHS/United States
- R01 HG002213/HG/NHGRI NIH HHS/United States
- M01 RR010284/RR/NCRR NIH HHS/United States
- UL1 TR001102/TR/NCATS NIH HHS/United States
- RR000533/RR/NCRR NIH HHS/United States
- HG006993/HG/NHGRI NIH HHS/United States
- RR010284/RR/NCRR NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
