Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression
- PMID: 26811325
- PMCID: PMC4854754
- DOI: 10.1158/2159-8290.CD-15-1183
Tumor Cell-Driven Extracellular Matrix Remodeling Drives Haptotaxis during Metastatic Progression
Abstract
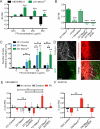
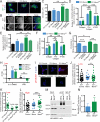
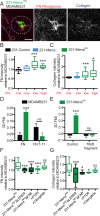
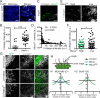
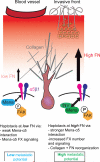
Fibronectin (FN) is a major component of the tumor microenvironment, but its role in promoting metastasis is incompletely understood. Here, we show that FN gradients elicit directional movement of breast cancer cells, in vitro and in vivo Haptotaxis on FN gradients requires direct interaction between α5β1 integrin and MENA, an actin regulator, and involves increases in focal complex signaling and tumor cell-mediated extracellular matrix (ECM) remodeling. Compared with MENA, higher levels of the prometastatic MENA(INV) isoform associate with α5, which enables 3-D haptotaxis of tumor cells toward the high FN concentrations typically present in perivascular space and in the periphery of breast tumor tissue. MENA(INV) and FN levels were correlated in two breast cancer cohorts, and high levels of MENA(INV) were significantly associated with increased tumor recurrence as well as decreased patient survival. Our results identify a novel tumor cell-intrinsic mechanism that promotes metastasis through ECM remodeling and ECM-guided directional migration.
Significance: Here, we provide new insight into how tumor cell:ECM interactions generate signals and structures that promote directed tumor cell migration, a critical component of metastasis. Our results identify a tumor cell-intrinsic mechanism driven by the actin regulatory protein MENA that promotes ECM remodeling and haptotaxis along FN gradients. Cancer Discov; 6(5); 516-31. ©2016 AACR.See related commentary by Santiago-Medina and Yang, p. 474This article is highlighted in the In This Issue feature, p. 461.
©2016 American Association for Cancer Research.
Figures


Comment in
-
MENA Promotes Tumor-Intrinsic Metastasis through ECM Remodeling and Haptotaxis.Cancer Discov. 2016 May;6(5):474-6. doi: 10.1158/2159-8290.CD-16-0231. Cancer Discov. 2016. PMID: 27138561 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

