BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors
- PMID: 26811382
- PMCID: PMC4813333
- DOI: 10.1242/dev.126656
BMP signaling and cellular dynamics during regeneration of airway epithelium from basal progenitors
Abstract
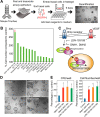
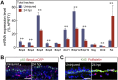
The pseudostratified epithelium of the lung contains ciliated and secretory luminal cells and basal stem/progenitor cells. To identify signals controlling basal cell behavior we screened factors that alter their self-renewal and differentiation in a clonal organoid (tracheosphere) assay. This revealed that inhibitors of the canonical BMP signaling pathway promote proliferation but do not affect lineage choice, whereas exogenous Bmp4 inhibits proliferation and differentiation. We therefore followed changes in BMP pathway components in vivo in the mouse trachea during epithelial regeneration from basal cells after injury. The findings suggest that BMP signaling normally constrains proliferation at steady state and this brake is released transiently during repair by the upregulation of endogenous BMP antagonists. Early in repair, the packing of epithelial cells along the basal lamina increases, but density is later restored by active extrusion of apoptotic cells. Systemic administration of the BMP antagonist LDN-193189 during repair initially increases epithelial cell number but, following the shedding phase, normal density is restored. Taken together, these results reveal crucial roles for both BMP signaling and cell shedding in homeostasis of the respiratory epithelium.
Keywords: Airway epithelium; Apoptosis; BMP signaling; Basal cells; Cell shedding; Homeostasis; Regeneration; SO2 injury.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





Similar articles
-
IL-6/STAT3 promotes regeneration of airway ciliated cells from basal stem cells.Proc Natl Acad Sci U S A. 2014 Sep 2;111(35):E3641-9. doi: 10.1073/pnas.1409781111. Epub 2014 Aug 18. Proc Natl Acad Sci U S A. 2014. PMID: 25136113 Free PMC article.
-
Wnt Signaling Regulates Airway Epithelial Stem Cells in Adult Murine Submucosal Glands.Stem Cells. 2016 Nov;34(11):2758-2771. doi: 10.1002/stem.2443. Epub 2016 Jul 11. Stem Cells. 2016. PMID: 27341073 Free PMC article.
-
p53 regulation by MDM2 contributes to self-renewal and differentiation of basal stem cells in mouse and human airway epithelium.FASEB J. 2021 Sep;35(9):e21816. doi: 10.1096/fj.202100638R. FASEB J. 2021. PMID: 34396583
-
BMP signaling in homeostasis, transformation and inflammatory response of intestinal epithelium.Sci China Life Sci. 2018 Jul;61(7):800-807. doi: 10.1007/s11427-018-9310-7. Epub 2018 May 29. Sci China Life Sci. 2018. PMID: 29855793 Review.
-
Airway epithelial repair, regeneration, and remodeling after injury in chronic obstructive pulmonary disease.Proc Am Thorac Soc. 2006 Nov;3(8):726-33. doi: 10.1513/pats.200605-126SF. Proc Am Thorac Soc. 2006. PMID: 17065381 Review.
Cited by
-
Building a human lung from pluripotent stem cells to model respiratory viral infections.Respir Res. 2024 Jul 15;25(1):277. doi: 10.1186/s12931-024-02912-0. Respir Res. 2024. PMID: 39010108 Free PMC article. Review.
-
Consecutive Hypoxia Decreases Expression of NOTCH3, HEY1, CC10, and FOXJ1 via NKX2-1 Downregulation and Intermittent Hypoxia-Reoxygenation Increases Expression of BMP4, NOTCH1, MKI67, OCT4, and MUC5AC via HIF1A Upregulation in Human Bronchial Epithelial Cells.Front Cell Dev Biol. 2020 Sep 4;8:572276. doi: 10.3389/fcell.2020.572276. eCollection 2020. Front Cell Dev Biol. 2020. PMID: 33015064 Free PMC article.
-
The diversity of adult lung epithelial stem cells and their niche in homeostasis and regeneration.Sci China Life Sci. 2021 Dec;64(12):2045-2059. doi: 10.1007/s11427-020-1902-3. Epub 2021 Apr 30. Sci China Life Sci. 2021. PMID: 33948870 Review.
-
Blocking TGF-β and BMP SMAD-dependent cell differentiation is a master key to expand all kinds of epithelial stem cells.Stem Cell Investig. 2016 Dec 8;3:88. doi: 10.21037/sci.2016.11.15. eCollection 2016. Stem Cell Investig. 2016. PMID: 28078268 Free PMC article. No abstract available.
-
Simple Models of Lung Development.Adv Exp Med Biol. 2023;1413:17-28. doi: 10.1007/978-3-031-26625-6_2. Adv Exp Med Biol. 2023. PMID: 37195524
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

