MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1
- PMID: 26811383
- PMCID: PMC4813332
- DOI: 10.1242/dev.126383
MEF2C regulates outflow tract alignment and transcriptional control of Tdgf1
Abstract
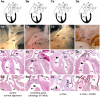
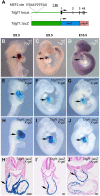
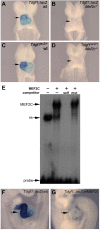
Congenital heart defects are the most common birth defects in humans, and those that affect the proper alignment of the outflow tracts and septation of the ventricles are a highly significant cause of morbidity and mortality in infants. A late differentiating population of cardiac progenitors, referred to as the anterior second heart field (AHF), gives rise to the outflow tract and the majority of the right ventricle and provides an embryological context for understanding cardiac outflow tract alignment and membranous ventricular septal defects. However, the transcriptional pathways controlling AHF development and their roles in congenital heart defects remain incompletely elucidated. Here, we inactivated the gene encoding the transcription factor MEF2C in the AHF in mice. Loss of Mef2c function in the AHF results in a spectrum of outflow tract alignment defects ranging from overriding aorta to double-outlet right ventricle and dextro-transposition of the great arteries. We identify Tdgf1, which encodes a Nodal co-receptor (also known as Cripto), as a direct transcriptional target of MEF2C in the outflow tract via an AHF-restricted Tdgf1 enhancer. Importantly, both the MEF2C and TDGF1 genes are associated with congenital heart defects in humans. Thus, these studies establish a direct transcriptional pathway between the core cardiac transcription factor MEF2C and the human congenital heart disease gene TDGF1. Moreover, we found a range of outflow tract alignment defects resulting from a single genetic lesion, supporting the idea that AHF-derived outflow tract alignment defects may constitute an embryological spectrum rather than distinct anomalies.
Keywords: Cripto; Enhancer; Heart development; MEF2; Mouse; Tdgf1.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

