Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary
- PMID: 26811385
- PMCID: PMC4813340
- DOI: 10.1242/dev.129627
Chinmo is sufficient to induce male fate in somatic cells of the adult Drosophila ovary
Abstract
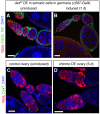
Sexual identity is continuously maintained in specific differentiated cell types long after sex determination occurs during development. In the adult Drosophila testis, the putative transcription factor Chronologically inappropriate morphogenesis (Chinmo) acts with the canonical male sex determinant DoublesexM (Dsx(M)) to maintain the male identity of somatic cyst stem cells and their progeny. Here we find that ectopic expression of chinmo is sufficient to induce a male identity in adult ovarian somatic cells, but it acts through a Dsx(M)-independent mechanism. Conversely, the feminization of the testis somatic stem cell lineage caused by loss of chinmo is enhanced by expression of the canonical female sex determinant Dsx(F), indicating that chinmo acts in parallel with the canonical sex determination pathway to maintain the male identity of testis somatic cells. Consistent with this finding, ectopic expression of female sex determinants in the adult testis disrupts tissue morphology. The miRNA let-7 downregulates chinmo in many contexts, and ectopic expression of let-7 in the adult testis is sufficient to recapitulate the chinmo loss-of-function phenotype, but we find no apparent phenotypes upon removal of let-7 in the adult ovary or testis. Our finding that chinmo is necessary and sufficient to promote a male identity in adult gonadal somatic cells suggests that the sexual identity of somatic cells can be reprogrammed in the adult Drosophila ovary as well as in the testis.
Keywords: Jak-STAT signaling; Niche; Ovary; Sex maintenance; Stem cell; Testis.
© 2016. Published by The Company of Biologists Ltd.
Conflict of interest statement
The authors declare no competing or financial interests.
Figures





Similar articles
-
Chinmo prevents transformer alternative splicing to maintain male sex identity.PLoS Genet. 2018 Feb 1;14(2):e1007203. doi: 10.1371/journal.pgen.1007203. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29389999 Free PMC article.
-
The Jak-STAT target Chinmo prevents sex transformation of adult stem cells in the Drosophila testis niche.Dev Cell. 2014 Nov 24;31(4):474-86. doi: 10.1016/j.devcel.2014.10.004. Epub 2014 Nov 13. Dev Cell. 2014. PMID: 25453558 Free PMC article.
-
chinmo Mutant fly testis stem cells switching sex farewell to maleness.Dev Cell. 2014 Nov 24;31(4):385-7. doi: 10.1016/j.devcel.2014.11.001. Epub 2014 Nov 24. Dev Cell. 2014. PMID: 25458005
-
Hedgehog in the Drosophila testis niche: what does it do there?Protein Cell. 2013 Sep;4(9):650-5. doi: 10.1007/s13238-013-3040-y. Epub 2013 Jun 26. Protein Cell. 2013. PMID: 23807635 Free PMC article. Review.
-
Double nexus--Doublesex is the connecting element in sex determination.Brief Funct Genomics. 2015 Nov;14(6):396-406. doi: 10.1093/bfgp/elv005. Epub 2015 Mar 22. Brief Funct Genomics. 2015. PMID: 25797692 Free PMC article. Review.
Cited by
-
Soma-germline communication drives sex maintenance in the Drosophila testis.Natl Sci Rev. 2024 Jun 22;11(8):nwae215. doi: 10.1093/nsr/nwae215. eCollection 2024 Aug. Natl Sci Rev. 2024. PMID: 39183747 Free PMC article.
-
Signal transduction in the early Drosophila follicle stem cell lineage.Curr Opin Insect Sci. 2020 Feb;37:39-48. doi: 10.1016/j.cois.2019.11.005. Epub 2020 Jan 30. Curr Opin Insect Sci. 2020. PMID: 32087562 Free PMC article. Review.
-
Chinmo prevents transformer alternative splicing to maintain male sex identity.PLoS Genet. 2018 Feb 1;14(2):e1007203. doi: 10.1371/journal.pgen.1007203. eCollection 2018 Feb. PLoS Genet. 2018. PMID: 29389999 Free PMC article.
-
Sex-converted testis somatic cells acquire female-specific behaviors and alter XY germline identity.Development. 2025 Aug 1;152(15):dev204785. doi: 10.1242/dev.204785. Epub 2025 Aug 7. Development. 2025. PMID: 40600835 Free PMC article.
-
Differentiating Drosophila female germ cells initiate Polycomb silencing by regulating PRC2-interacting proteins.Elife. 2020 Aug 10;9:e56922. doi: 10.7554/eLife.56922. Elife. 2020. PMID: 32773039 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

