Biologic-free mechanically induced muscle regeneration
- PMID: 26811474
- PMCID: PMC4760832
- DOI: 10.1073/pnas.1517517113
Biologic-free mechanically induced muscle regeneration
Abstract
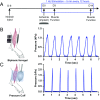
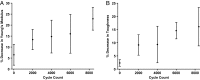
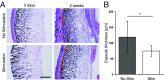
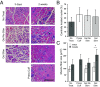
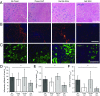
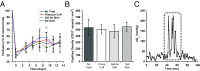
Severe skeletal muscle injuries are common and can lead to extensive fibrosis, scarring, and loss of function. Clinically, no therapeutic intervention exists that allows for a full functional restoration. As a result, both drug and cellular therapies are being widely investigated for treatment of muscle injury. Because muscle is known to respond to mechanical loading, we investigated instead whether a material system capable of massage-like compressions could promote regeneration. Magnetic actuation of biphasic ferrogel scaffolds implanted at the site of muscle injury resulted in uniform cyclic compressions that led to reduced fibrous capsule formation around the implant, as well as reduced fibrosis and inflammation in the injured muscle. In contrast, no significant effect of ferrogel actuation on muscle vascularization or perfusion was found. Strikingly, ferrogel-driven mechanical compressions led to enhanced muscle regeneration and a ∼threefold increase in maximum contractile force of the treated muscle at 2 wk compared with no-treatment controls. Although this study focuses on the repair of severely injured skeletal muscle, magnetically stimulated bioagent-free ferrogels may find broad utility in the field of regenerative medicine.
Keywords: fibrous capsule; immunomodulation; magnetic hydrogel; massage-mimetic; mechano-therapy.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Turner NJ, Badylak SF. Regeneration of skeletal muscle. Cell Tissue Res. 2012;347(3):759–774. - PubMed
-
- Järvinen TAHJ, Järvinen TLN, Kääriäinen M, Kalimo H, Järvinen M. Muscle injuries: Biology and treatment. Am J Sports Med. 2005;33(5):745–764. - PubMed
-
- Ma CH, et al. Reconstruction of upper extremity large soft-tissue defects using pedicled latissimus dorsi muscle flaps--technique illustration and clinical outcomes. Injury. 2008;39(Suppl 4):67–74. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

