TSGΔ154-1054 splice variant increases TSG101 oncogenicity by inhibiting its E3-ligase-mediated proteasomal degradation
- PMID: 26811492
- PMCID: PMC4884989
- DOI: 10.18632/oncotarget.6973
TSGΔ154-1054 splice variant increases TSG101 oncogenicity by inhibiting its E3-ligase-mediated proteasomal degradation
Abstract
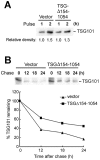
Tumor susceptibility gene 101 (TSG101) elicits an array of cellular functions, including promoting cytokinesis, cell cycle progression and proliferation, as well as facilitating endosomal trafficking and viral budding. TSG101 protein is highly and aberrantly expressed in various human cancers. Specifically, a TSG101 splicing variant missing nucleotides 154 to 1054 (TSGΔ154-1054), which is linked to progressive tumor-stage and metastasis, has puzzled investigators for more than a decade. TSG101-associated E3 ligase (Tal)- and MDM2-mediated proteasomal degradation are the two major routes for posttranslational regulation of the total amount of TSG101. We reveal that overabundance of TSG101 results from TSGΔ154-1054 stabilizing the TSG101 protein by competitively binding to Tal, but not MDM2, thereby perturbing the Tal interaction with TSG101 and impeding subsequent polyubiquitination and proteasomal degradation of TSG101. TSGΔ154-1054 therefore specifically enhances TSG101-stimulated cell proliferation, clonogenicity, and tumor growth in nude mice. This finding shows the functional significance of TSGΔ154-1054 in preventing the ubiquitin-proteasome proteolysis of TSG101, which increases tumor malignancy and hints at its potential as a therapeutic target in cancer treatment.
Keywords: TSG101; alternative splicing; nasopharyngeal carcinoma; tumorigenesis; ubiquitination.
Conflict of interest statement
There is no conflict of interest.
Figures




Similar articles
-
Cancer-Specifically Re-Spliced TSG101 mRNA Promotes Invasion and Metastasis of Nasopharyngeal Carcinoma.Int J Mol Sci. 2019 Feb 12;20(3):773. doi: 10.3390/ijms20030773. Int J Mol Sci. 2019. PMID: 30759747 Free PMC article.
-
Tsg101 mimicry of canonical E2 enzymes underlies its role in ubiquitin signaling.Proc Natl Acad Sci U S A. 2025 Jan 7;122(1):e2419542121. doi: 10.1073/pnas.2419542121. Epub 2024 Dec 31. Proc Natl Acad Sci U S A. 2025. PMID: 39739800 Free PMC article.
-
Characterization of the GNMT-HectH9-PREX2 tripartite relationship in the pathogenesis of hepatocellular carcinoma.Int J Cancer. 2017 May 15;140(10):2284-2297. doi: 10.1002/ijc.30652. Int J Cancer. 2017. PMID: 28205209
-
Role of TSG101 in cancer.Front Biosci (Landmark Ed). 2013 Jan 1;18(1):279-88. doi: 10.2741/4099. Front Biosci (Landmark Ed). 2013. PMID: 23276921 Review.
-
Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review.
Cited by
-
The Exon Junction Complex Core Represses Cancer-Specific Mature mRNA Re-splicing: A Potential Key Role in Terminating Splicing.Int J Mol Sci. 2021 Jun 17;22(12):6519. doi: 10.3390/ijms22126519. Int J Mol Sci. 2021. PMID: 34204574 Free PMC article.
-
Adding Some "Splice" to Stress Eating: Autophagy, ESCRT and Alternative Splicing Orchestrate the Cellular Stress Response.Genes (Basel). 2021 Jul 31;12(8):1196. doi: 10.3390/genes12081196. Genes (Basel). 2021. PMID: 34440370 Free PMC article. Review.
-
Epstein-Barr Virus Enhances Cancer-Specific Aberrant Splicing of TSG101 Pre-mRNA.Int J Mol Sci. 2022 Feb 24;23(5):2516. doi: 10.3390/ijms23052516. Int J Mol Sci. 2022. PMID: 35269659 Free PMC article.
-
Cancer-Specifically Re-Spliced TSG101 mRNA Promotes Invasion and Metastasis of Nasopharyngeal Carcinoma.Int J Mol Sci. 2019 Feb 12;20(3):773. doi: 10.3390/ijms20030773. Int J Mol Sci. 2019. PMID: 30759747 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

