Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease
- PMID: 26811520
- PMCID: PMC4872017
- DOI: 10.1200/JCO.2015.64.5929
Allogeneic T Cells That Express an Anti-CD19 Chimeric Antigen Receptor Induce Remissions of B-Cell Malignancies That Progress After Allogeneic Hematopoietic Stem-Cell Transplantation Without Causing Graft-Versus-Host Disease
Abstract
Purpose: Progressive malignancy is the leading cause of death after allogeneic hematopoietic stem-cell transplantation (alloHSCT). After alloHSCT, B-cell malignancies often are treated with unmanipulated donor lymphocyte infusions (DLIs) from the transplant donor. DLIs frequently are not effective at eradicating malignancy and often cause graft-versus-host disease, a potentially lethal immune response against normal recipient tissues.
Methods: We conducted a clinical trial of allogeneic T cells genetically engineered to express a chimeric antigen receptor (CAR) targeting the B-cell antigen CD19. Patients with B-cell malignancies that had progressed after alloHSCT received a single infusion of CAR T cells. No chemotherapy or other therapies were administered. The T cells were obtained from each recipient's alloHSCT donor.
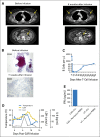
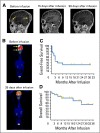
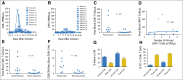
Results: Eight of 20 treated patients obtained remission, which included six complete remissions (CRs) and two partial remissions. The response rate was highest for acute lymphoblastic leukemia, with four of five patients obtaining minimal residual disease-negative CR. Responses also occurred in chronic lymphocytic leukemia and lymphoma. The longest ongoing CR was more than 30 months in a patient with chronic lymphocytic leukemia. New-onset acute graft-versus-host disease after CAR T-cell infusion developed in none of the patients. Toxicities included fever, tachycardia, and hypotension. Peak blood CAR T-cell levels were higher in patients who obtained remissions than in those who did not. Programmed cell death protein-1 expression was significantly elevated on CAR T cells after infusion. Presence of blood B cells before CAR T-cell infusion was associated with higher postinfusion CAR T-cell levels.
Conclusion: Allogeneic anti-CD19 CAR T cells can effectively treat B-cell malignancies that progress after alloHSCT. The findings point toward a future when antigen-specific T-cell therapies will play a central role in alloHSCT.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures

References
-
- Pavletic SZ, Kumar S, Mohty M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: Report from the Committee on the Epidemiology and Natural History of Relapse Following Allogeneic Cell Transplantation. Biol Blood Marrow Transplant. 2010;16:871–890. - PMC - PubMed
-
- Spyridonidis A, Labopin M, Schmid C, et al. Immunotherapy Subcommittee of Acute Leukemia Working Party Outcomes and prognostic factors of adults with acute lymphoblastic leukemia who relapse after allogeneic hematopoietic cell transplantation. An analysis on behalf of the Acute Leukemia Working Party of EBMT. Leukemia. 2012;26:1211–1217. - PubMed
-
- Thomson KJ, Morris EC, Bloor A, et al. Favorable long-term survival after reduced-intensity allogeneic transplantation for multiple-relapse aggressive non-Hodgkin’s lymphoma. J Clin Oncol. 2009;27:426–432. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

