Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection
- PMID: 26811528
- PMCID: PMC4933133
- DOI: 10.1200/JCO.2015.64.0094
Improved Axillary Evaluation Following Neoadjuvant Therapy for Patients With Node-Positive Breast Cancer Using Selective Evaluation of Clipped Nodes: Implementation of Targeted Axillary Dissection
Abstract
Purpose: Placing clips in nodes with biopsy-confirmed metastasis before initiating neoadjuvant therapy allows for evaluation of response in breast cancer. Our goal was to determine if pathologic changes in clipped nodes reflect the status of the nodal basin and if targeted axillary dissection (TAD), which includes sentinel lymph node dissection (SLND) and selective localization and removal of clipped nodes, improves the false-negative rate (FNR) compared with SLND alone.
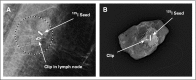
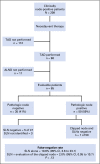
Methods: A prospective study of patients with biopsy-confirmed nodal metastases with a clip placed in the sampled node was performed. After neoadjuvant therapy, patients underwent axillary surgery and the pathology of the clipped node was compared with other nodes. Patients undergoing TAD had SLND and selective removal of the clipped node using iodine-125 seed localization. The FNR was determined in patients undergoing complete axillary lymphadenectomy (ALND).
Results: Of 208 patients enrolled in this study, 191 underwent ALND, with residual disease identified in 120 (63%). The clipped node revealed metastases in 115 patients, resulting in an FNR of 4.2% (95% CI, 1.4 to 9.5) for the clipped node. In patients undergoing SLND and ALND (n = 118), the FNR was 10.1% (95% CI, 4.2 to 19.8), which included seven false-negative events in 69 patients with residual disease. Adding evaluation of the clipped node reduced the FNR to 1.4% (95% CI, 0.03 to 7.3; P = .03). The clipped node was not retrieved as an SLN in 23% (31 of 134) of patients, including six with negative SLNs but metastasis in the clipped node. TAD followed by ALND was performed in 85 patients, with an FNR of 2.0% (1 of 50; 95% CI, 0.05 to 10.7).
Conclusion: Marking nodes with biopsy-confirmed metastatic disease allows for selective removal and improves pathologic evaluation for residual nodal disease after chemotherapy.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at
Figures

Comment in
-
Can a seed-sized tool from Texas spare clinically node positive breast cancer patients from a complete axillary dissection?Gland Surg. 2016 Aug;5(4):450-2. doi: 10.21037/gs.2016.07.02. Gland Surg. 2016. PMID: 27562286 Free PMC article. No abstract available.
References
-
- Buzdar AU, Ibrahim NK, Francis D, et al. Significantly higher pathologic complete remission rate after neoadjuvant therapy with trastuzumab, paclitaxel, and epirubicin chemotherapy: Results of a randomized trial in human epidermal growth factor receptor 2-positive operable breast cancer. J Clin Oncol. 2005;23:3676–3685. - PubMed
-
- Hennessy BT, Hortobagyi GN, Rouzier R, et al. Outcome after pathologic complete eradication of cytologically proven breast cancer axillary node metastases following primary chemotherapy. J Clin Oncol. 2005;23:9304–9311. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

