Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database
- PMID: 26811529
- PMCID: PMC4872008
- DOI: 10.1200/JCO.2015.63.0558
Impact of Patient Factors on Recurrence Risk and Time Dependency of Oxaliplatin Benefit in Patients With Colon Cancer: Analysis From Modern-Era Adjuvant Studies in the Adjuvant Colon Cancer End Points (ACCENT) Database
Abstract
Purpose: Fluorouracil plus leucovorin (FU + LV) adjuvant chemotherapy reduced the risk of recurrence and death across all time points in a pooled analysis of 20,898 patients with colon cancer from 18 randomized studies. The impact of oxaliplatin added to FU + LV on the time course of recurrence and survival remains unknown.
Patients and methods: A total of 12,233 patients enrolled to the randomized trials C-07, C-08, N0147, MOSAIC (Adjuvant Treatment of Colon Cancer), and XELOXA (Adjuvant XELOX) were pooled to examine the impact of oxaliplatin and tumor-specific factors on the time course of recurrence and death. For each end point, continuous-time risk was modeled over 6 years post treatment in all oxaliplatin-treated patients and patients concurrently randomized to FU + LV with or without oxaliplatin; the latter analyses supported time-dependent treatment comparisons.
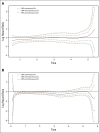
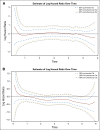
Results: Addition of oxaliplatin significantly reduced the risk of recurrence within the first 14 months post treatment for patients with stage II disease and within the first 4 years for patients with stage III disease. Oxaliplatin also significantly reduced risk of death from 2 to 6 years post treatment for patients with stage III disease, with no differences in timing of outcomes between treatment groups (ie, oxaliplatin did not simply postpone recurrence or death compared with FU + LV alone). Patients with stage II disease receiving oxaliplatin did not exhibit a significant reduction in risk of death in the first 6 years post treatment. Recurrence risk peaked near 14 months for both treatments, and risk of recurrence and death increased with increased tumor and nodal burden.
Conclusions: These analyses support the addition of oxaliplatin to fluoropyrimidine-based adjuvant therapy in patients with stage III disease and underscore the need for adequate surveillance of patients with colon cancer during the first 3 years after adjuvant therapy.
© 2016 by American Society of Clinical Oncology.
Conflict of interest statement
Authors' disclosures of potential conflicts of interest are found in the article online at www.jco.org. Author contributions are found at the end of this article.
Figures




References
-
- Ferlay J, Soerjomataram I, Ervik M, et al. GLOBOCAN 2012 v1.0, Cancer incidence and mortality worldwide: IARC CancerBase No. 11 [Internet] 2013. International Agency for Research on Cancer 2013.
-
- André T, Boni C, Mounedji-Boudiaf L, et al. Multicenter International Study of Oxaliplatin/5-Fluorouracil/Leucovorin in the Adjuvant Treatment of Colon Cancer (MOSAIC) Investigators Oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment for colon cancer. N Engl J Med. 2004;350:2343–2351. - PubMed
-
- André T, Boni C, Navarro M, et al. Improved overall survival with oxaliplatin, fluorouracil, and leucovorin as adjuvant treatment in stage II or III colon cancer in the MOSAIC trial. J Clin Oncol. 2009;27:3109–3116. - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

