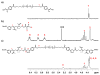
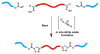
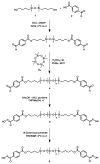
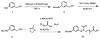Rapid Metal -free Macromolecular Coupling via in situ Nitrile Oxide-Activated Alkene Cycloaddition
- PMID: 26811566
- PMCID: PMC4723422
- DOI: 10.1002/pola.27371
Rapid Metal -free Macromolecular Coupling via in situ Nitrile Oxide-Activated Alkene Cycloaddition
Abstract
Nitrile oxide 1,3 dipolar cycloaddition is a simple and powerful coupling methodology. However, the self-dimerization of nitrile oxides has prevented the widespread use of this strategy for macromolecular coupling. By combining an in situ nitrile oxide generation with a highly reactive activated dipolarophile, we have overcome these obstacles and present a metal-free macromolecular coupling strategy for the modular synthesis of several ABA triblock copolymers. Nitrile oxides were generated in situ from chloroxime terminated poly(dimethylsiloxane) B-blocks and coupled with several distinct hydrophilic (poly(2-methyloxazoline) and poly(ethylene glycol)), and poly(N-isopropylacrylamide) or hydrophobic (poly(L-lactide) A-blocks terminated in activated dipolarophiles in a rapid fashion with high yield. This methodology overcomes many drawbacks of previously reported metal-free methods due to its rapid kinetics, versatility, scalability, and ease of introduction of necessary functionality. Nitrile oxide cycloaddition should find use as an attractive macromolecular coupling strategy for the synthesis of biocompatible polymeric nanostructures.
Keywords: 1,3-dipolar cycloaddition; nitrile oxide; poly(2-methyloxazoline) (PMOXA); poly(L-lactide) (PLA); poly(N-isopropylacrylamide) (PNIPAM); poly(dimethylsiloxane) (PDMS); poly(ethylene glycol) (PEG); triblock copolymers.
Figures
Similar articles
-
Stealth polymeric vesicles via metal-free click coupling.Biomacromolecules. 2013 Sep 9;14(9):2996-3000. doi: 10.1021/bm400940h. Epub 2013 Aug 21. Biomacromolecules. 2013. PMID: 23952743 Free PMC article.
-
Nitrile Oxide, Alkenes, Dipolar Cycloaddition, Isomerization and Metathesis Involved in the Syntheses of 2-Isoxazolines.Molecules. 2023 Mar 10;28(6):2547. doi: 10.3390/molecules28062547. Molecules. 2023. PMID: 36985520 Free PMC article. Review.
-
ABA-triblock copolymers from biodegradable polyester A-blocks and hydrophilic poly(ethylene oxide) B-blocks as a candidate for in situ forming hydrogel delivery systems for proteins.Adv Drug Deliv Rev. 2002 Jan 17;54(1):99-134. doi: 10.1016/s0169-409x(01)00244-7. Adv Drug Deliv Rev. 2002. PMID: 11755708 Review.
-
Environmentally Benign and User-Friendly In Situ Generation of Nitrile Imines from Hydrazones for 1,3-Dipolar Cycloaddition.J Org Chem. 2022 Aug 5;87(15):10550-10554. doi: 10.1021/acs.joc.2c01391. Epub 2022 Jul 22. J Org Chem. 2022. PMID: 35866673
-
Thermoresponsive physical hydrogels of poly(lactic acid)/poly(ethylene glycol) stereoblock copolymers tuned by stereostructure and hydrophobic block sequence.Soft Matter. 2016 May 18;12(20):4628-37. doi: 10.1039/c6sm00517a. Soft Matter. 2016. PMID: 27121732
Cited by
-
Convenient Synthesis of Pyrazolo[4',3':5,6]pyrano[4,3-c][1,2]oxazoles via Intramolecular Nitrile Oxide Cycloaddition.Molecules. 2021 Sep 15;26(18):5604. doi: 10.3390/molecules26185604. Molecules. 2021. PMID: 34577075 Free PMC article.
References
-
- Brinkhuis RP, Rutjes FPJT, van Hest JCM. Polym Chem. 2011;2:1449–1462.
-
- Liu GY, Chen CJ, Ji J. Soft Matter. 2012;8:8811–8821.
-
- Lee JS, Feijen J. J Control Release. 2012;161:473–483. - PubMed
-
- LoPresti C, Lomas H, Massignani M, Smart T, Battaglia G. J Mater Chem. 2009;19:3576–3590.
-
- Discher BM, Won YY, Ege DS, Lee JCM, Bates FS, Discher DE, Hammer DA. Science. 1999;284:1143–1146. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources