Advances in cryoablation for pancreatic cancer
- PMID: 26811625
- PMCID: PMC4716077
- DOI: 10.3748/wjg.v22.i2.790
Advances in cryoablation for pancreatic cancer
Abstract
Pancreatic carcinoma is a common cancer of the digestive system with a poor prognosis. It is characterized by insidious onset, rapid progression, a high degree of malignancy and early metastasis. At present, radical surgery is considered the only curative option for treatment, however, the majority of patients with pancreatic cancer are diagnosed too late to undergo surgery. The sensitivity of pancreatic cancer to chemotherapy or radiotherapy is also poor. As a result, there is no standard treatment for patients with advanced pancreatic cancer. Cryoablation is generally considered to be an effective palliative treatment for pancreatic cancer. It has the advantages of minimal invasion and improved targeting, and is potentially safe with less pain to the patients. It is especially suitable in patients with unresectable pancreatic cancer. However, our initial findings suggest that cryotherapy combined with 125-iodine seed implantation, immunotherapy or various other treatments for advanced pancreatic cancer can improve survival in patients with unresectable or metastatic pancreatic cancer. Although these findings require further in-depth study, the initial results are encouraging. This paper reviews the safety and efficacy of cryoablation, including combined approaches, in the treatment of pancreatic cancer.
Keywords: Combination therapy; Cryoablation; Cryoimmunotherapy; Pancreatic cancer.
Figures
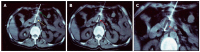








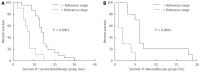
References
-
- Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. - PubMed
-
- Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61:69–90. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;62:10–29. - PubMed
-
- Siegel R, Naishadham D, Jemal A. Cancer statistics, 2013. CA Cancer J Clin. 2013;63:11–30. - PubMed
-
- Wilkowski R, Thoma M, Bruns C, Wagner A, Heinemann V. Chemoradiotherapy with gemcitabine and continuous 5-FU in patients with primary inoperable pancreatic cancer. JOP. 2006;7:349–360. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

