Safety and efficacy of paliperidone extended-release in Chinese patients with schizophrenia: a 24-week, open-label extension of a randomized, double-blind, placebo-controlled study
- PMID: 26811679
- PMCID: PMC4712970
- DOI: 10.2147/NDT.S88875
Safety and efficacy of paliperidone extended-release in Chinese patients with schizophrenia: a 24-week, open-label extension of a randomized, double-blind, placebo-controlled study
Abstract
Objectives: The long-term safety, tolerability, and efficacy of paliperidone extended-release (ER) were evaluated in Chinese patients with schizophrenia.
Methods: Patients (aged ≥18 years) with schizophrenia (Diagnostic and Statistical Manual of Mental Disorders, 4th edition criteria) who had completed run-in (8-week), stabilization (6-week), and double-blind (DB) phases (variable) of a phase-3, placebo-controlled study entered this 24-week, open-label extension (OLE) study. These patients, who had either experienced a relapse or remained relapse-free through DB phase of the study, were treated with flexible-dose paliperidone-ER (3-12 mg/day) during the OLE phase. Major safety evaluations included treatment-emergent adverse events (TEAEs) and extrapyramidal symptoms. Efficacy endpoints included changes in Positive and Negative Syndrome Scale total score, Clinical Global Impression-Severity scale, and Personal and Social Performance scale from OLE baseline to OLE endpoint.
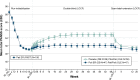
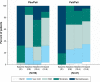
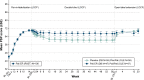
Results: Out of 106 patients who entered the OLE phase (placebo: 59, paliperidone-ER: 47), a total of 85 (80%) completed it. Thirty-five (33%) patients experienced at least one TEAE; most common were akathisia, somnolence, nasopharyngitis, and constipation (3.8% each). Serious TEAEs were noted in two patients (completed suicide; schizophrenia worsening). No TEAEs with an onset during the OLE phase led to discontinuation. Extrapyramidal symptoms related-TEAEs were reported in eight (7.5%) patients. Mean (standard deviation) changes in Positive and Negative Syndrome Scale total scores (-10.4 [13.2]), Clinical Global Impression-Severity scores (-0.6 [0.96]) and Personal and Social Performance scores (7.4 [13.2]) from OLE baseline to OLE endpoint showed patients who had been treated with placebo during the DB phase experienced more pronounced improvements.
Conclusion: In this OLE study, flexibly dosed paliperidone-ER (3-12 mg/day) was tolerable and efficacious in Chinese patients with schizophrenia.
Keywords: CGI-S score; PANSS score; PSP score; paliperidone.
Figures
References
-
- Li H, Rui Q, Ning X, Xu H, Gu NA. Comparative study of paliperidone palmitate and risperidone long-acting injectable therapy in schizophrenia. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35(4):1002–1008. - PubMed
-
- Leucht S, Cipriani A, Spineli L, et al. Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet. 2013;382(9896):951–962. - PubMed
-
- Canuso CM, Battisti WP. Paliperidone extended-release: a review of efficacy and tolerability in schizophrenia, schizoaffective disorder and bipolar mania. Expert Opin Pharmacother. 2010;11(15):2557–2567. - PubMed
-
- Versola-Russo JM. Cultural and demographic factors of schizophrenia. The International Journal of Psychosocial Rehabilitation. 2006;10:89–103.
-
- Williams DR, Earl TR. Commentary: Race and mental health – more questions than answers. Int J Epidemiol. 2007;36(4):758–760. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

