Modelling the Tox21 10 K chemical profiles for in vivo toxicity prediction and mechanism characterization
- PMID: 26811972
- PMCID: PMC4777217
- DOI: 10.1038/ncomms10425
Modelling the Tox21 10 K chemical profiles for in vivo toxicity prediction and mechanism characterization
Abstract
Target-specific, mechanism-oriented in vitro assays post a promising alternative to traditional animal toxicology studies. Here we report the first comprehensive analysis of the Tox21 effort, a large-scale in vitro toxicity screening of chemicals. We test ∼ 10,000 chemicals in triplicates at 15 concentrations against a panel of nuclear receptor and stress response pathway assays, producing more than 50 million data points. Compound clustering by structure similarity and activity profile similarity across the assays reveals structure-activity relationships that are useful for the generation of mechanistic hypotheses. We apply structural information and activity data to build predictive models for 72 in vivo toxicity end points using a cluster-based approach. Models based on in vitro assay data perform better in predicting human toxicity end points than animal toxicity, while a combination of structural and activity data results in better models than using structure or activity data alone. Our results suggest that in vitro activity profiles can be applied as signatures of compound mechanism of toxicity and used in prioritization for more in-depth toxicological testing.
Figures
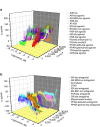
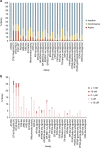

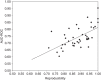
References
-
- NRC Toxicity Testing in the 21st Century: A Vision and a Strategy The National Academies Press (2007).
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

