Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface
- PMID: 26812077
- PMCID: PMC6278816
- DOI: 10.1016/j.vaccine.2016.01.024
Immunization with the Haemophilus ducreyi trimeric autotransporter adhesin DsrA with alum, CpG or imiquimod generates a persistent humoral immune response that recognizes the bacterial surface
Abstract
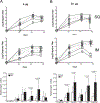
The Ducreyi serum resistance A (DsrA) protein of Haemophilus ducreyi belongs to a large family of multifunctional outer membrane proteins termed trimeric autotransporter adhesins responsible for resistance to the bactericidal activity of human complement (serum resistance), agglutination and adhesion. The ability of DsrA to confer serum resistance and bind extracellular matrix proteins lies in its N-terminal passenger domain. We have previously reported that immunization with a recombinant form of the passenger domain of DsrA, rNT-DsrA, in complete/incomplete Freund's adjuvant, protects against a homologous challenge in swine. We present herein the results of an immunogenicity study in mice aimed at investigating the persistence, type of immune response, and the effect of immunization route and adjuvants on surrogates of protection. Our results indicate that a 20 μg dose of rNT-DsrA administered with alum elicited antisera with comparable bacterial surface reactivity to that obtained with complete/incomplete Freund's adjuvant. At that dose, high titers and bacterial surface reactivity persisted for 211 days after the first immunization. Administration of rNT-DsrA with CpG or imiquimod as adjuvants elicited a humoral response with similar quantity and quality of antibodies (Abs) as seen with Freund's adjuvant. Furthermore, intramuscular administration of rNT-DsrA elicited high-titer Abs with significantly higher reactivity to the bacterial surface than those obtained with subcutaneous immunization. All rNT-DsrA/adjuvant combinations tested, save CpG, elicited a Th2-type response. Taken together, these findings show that a 20 μg dose of rNT-DsrA administered with the adjuvants alum, CpG or imiquimod elicits high-quality Abs with reactivity to the bacterial surface that could protect against an H. ducreyi infection.
Keywords: DsrA; Haemophilus ducreyi; Trimeric autotransporter adhesin; Vaccine.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Conflict of interest statement
CONFLICT OF INTEREST STATEMENT
The authors declare no competing personal or financial interests.
Figures
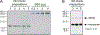

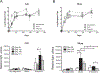


References
-
- Ussher JE, Wilson E, Campanella S, Taylor SL, Roberts SA. Haemophilus ducreyi causing chronic skin ulceration in children visiting Samoa. Clin Infect Dis 2007;44:e85–7. - PubMed
-
- McBride WJ, Hannah RC, Le Cornec GM, Bletchly C. Cutaneous chancroid in a visitor from Vanuatu. Australas J Dermatol 2008;49:98–9. - PubMed
-
- Peel TN, Bhatti D, De Boer JC, Stratov I, Spelman DW. Chronic cutaneous ulcers secondary to Haemophilus ducreyi infection. Med J Aust 2010;192:348–50. - PubMed
-
- Mitja O, Lukehart SA, Pokowas G, Moses P, Kapa A, Godornes C, et al. Haemophilus ducreyi as a cause of skin ulcers in children from a yaws-endemic area of Papua New Guinea: a prospective cohort study. Lancet Glob Health 2014;2:e235–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

