The Halogen Bond
- PMID: 26812185
- PMCID: PMC4768247
- DOI: 10.1021/acs.chemrev.5b00484
The Halogen Bond
Abstract
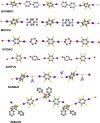
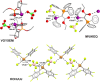
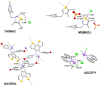
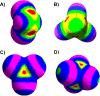
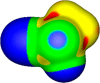
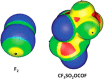
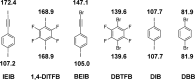
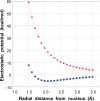
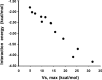
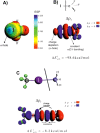
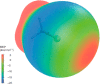
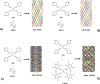
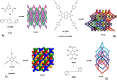
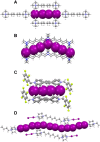
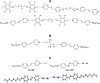
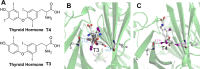
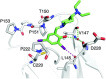
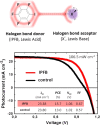
The halogen bond occurs when there is evidence of a net attractive interaction between an electrophilic region associated with a halogen atom in a molecular entity and a nucleophilic region in another, or the same, molecular entity. In this fairly extensive review, after a brief history of the interaction, we will provide the reader with a snapshot of where the research on the halogen bond is now, and, perhaps, where it is going. The specific advantages brought up by a design based on the use of the halogen bond will be demonstrated in quite different fields spanning from material sciences to biomolecular recognition and drug design.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

















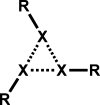






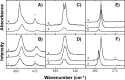

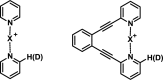
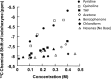

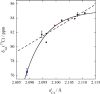

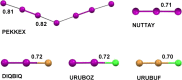


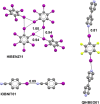


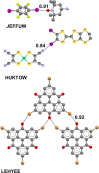
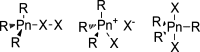




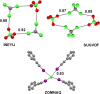
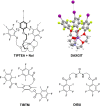

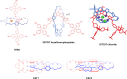
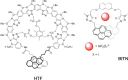
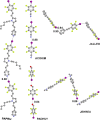
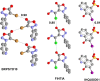
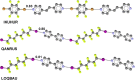
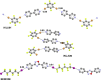
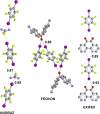

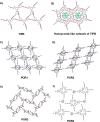
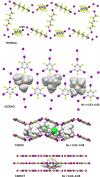


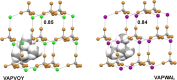




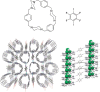
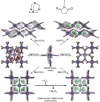

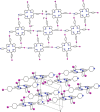
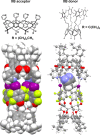


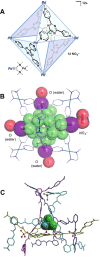


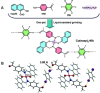

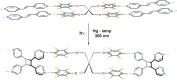

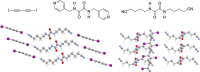




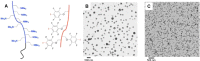








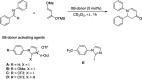
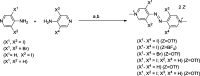

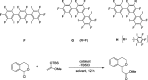
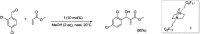
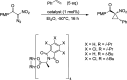
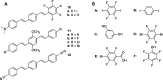
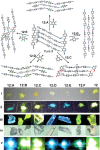





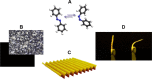








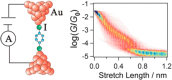
References
-
- Hantzsch A. Die Chromoisomerie Der P-Dioxy-Terepthalsaure Derivate Als Phenol-Enol-Isomerie. Ber. Dtsch. Chem. Ges. 1915, 48, 797–816. 10.1002/cber.191504801104. - DOI
-
- Nakamoto K.; Margoshes M.; Rundle R. E. Stretching Frequencies as a Function of Distances in Hydrogen Bonds. J. Am. Chem. Soc. 1955, 77, 6480–6486. 10.1021/ja01629a013. - DOI
-
- Schleyer P. V. R.; West R. Comparison of Covalently Bonded Electronegative Atoms as Proton Acceptor Groups in Hydrogen Bonding. J. Am. Chem. Soc. 1959, 81, 3164–3165. 10.1021/ja01521a084. - DOI
-
- Halogen Bonding. Fundamentals and Applications; Metrangolo P., Resnati G., Eds.; Springer-Verlag: Berlin, Heidelberg, 2008.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

