Real-Time PCR for Measles Virus Detection on Clinical Specimens with Negative IgM Result in Morocco
- PMID: 26812434
- PMCID: PMC4727926
- DOI: 10.1371/journal.pone.0147154
Real-Time PCR for Measles Virus Detection on Clinical Specimens with Negative IgM Result in Morocco
Abstract
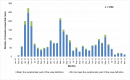
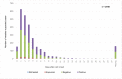
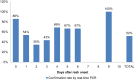
Since the confirmation of measles cases represents an important indicator regarding the performance of the measles-elimination program, the aim of this study was to evaluate the effectiveness of the routine procedures followed in Morocco for the laboratory confirmation of measles cases. Suspected cases reported between January 2010 and December 2012 were assessed for the timeliness of the sample collection, occurrence of measles clinical symptoms, and the results of the laboratory diagnoses. For 88% of the 2,708 suspected cases, a clinical specimen was collected within 7d of rash onset, of which 50% were IgM-positive and 2.6% were equivocal. The measles symptoms were reported in 91.4% of the cases; the occurrence of symptoms showed a positive association with the serological results (odds ratio [OR] = 2.9883, 95% confidence interval [CI] 2.2238-4.0157). Of the negative samples, 52% (n = 116) tested positive by real-time polymerase chain reaction (PCR). These results are in favor of using molecular detection to complement serological diagnosis in the context of measles surveillance approach in Morocco. In addition, the introduction of additional laboratory methods for differential diagnosis is required for the final classification of suspected cases with maculopapular rash and fever in the context of the measles elimination program.
Conflict of interest statement
Figures
Similar articles
-
Importance of real-time RT-PCR to supplement the laboratory diagnosis in the measles elimination program in China.PLoS One. 2018 Nov 30;13(11):e0208161. doi: 10.1371/journal.pone.0208161. eCollection 2018. PLoS One. 2018. PMID: 30500842 Free PMC article.
-
Combination of reverse transcriptase PCR analysis and immunoglobulin M detection on filter paper blood samples allows diagnostic and epidemiological studies of measles.J Clin Microbiol. 2001 Jan;39(1):270-3. doi: 10.1128/JCM.39.1.270-273.2001. J Clin Microbiol. 2001. PMID: 11136782 Free PMC article.
-
Laboratory diagnosis of vaccine-associated measles in Zhejiang Province, China.J Microbiol Immunol Infect. 2017 Oct;50(5):578-585. doi: 10.1016/j.jmii.2015.10.004. Epub 2015 Nov 19. J Microbiol Immunol Infect. 2017. PMID: 26698687
-
The challenges and strategies for laboratory diagnosis of measles in an international setting.J Infect Dis. 2003 May 15;187 Suppl 1:S283-90. doi: 10.1086/368040. J Infect Dis. 2003. PMID: 12721927 Review.
-
Rapid diagnostic tests to address challenges for global measles surveillance.Curr Opin Virol. 2020 Apr;41:77-84. doi: 10.1016/j.coviro.2020.05.007. Epub 2020 Jun 29. Curr Opin Virol. 2020. PMID: 32615510 Free PMC article. Review.
Cited by
-
Clinicoepidemiological profile of measles in adults: A study from semi-urban area in South India.Med J Armed Forces India. 2025 May-Jun;81(3):307-313. doi: 10.1016/j.mjafi.2024.03.004. Epub 2024 Jun 8. Med J Armed Forces India. 2025. PMID: 40463610
-
Laboratory methods supporting measles surveillance in Queensland, Australia, 2010-2017.Access Microbiol. 2020 Jan 27;2(3):acmi000093. doi: 10.1099/acmi.0.000093. eCollection 2020. Access Microbiol. 2020. PMID: 32974570 Free PMC article.
-
Importance of real-time RT-PCR to supplement the laboratory diagnosis in the measles elimination program in China.PLoS One. 2018 Nov 30;13(11):e0208161. doi: 10.1371/journal.pone.0208161. eCollection 2018. PLoS One. 2018. PMID: 30500842 Free PMC article.
References
-
- Featherstone D, Brown D, Sanders R. Development of the global measles laboratory network. J Infect Dis [Internet]. 2003. [cited 2013 Nov 2];187(Supplement 1):S264–9. Available: http://jid.oxfordjournals.org/content/187/Supplement_1/S264.short - PubMed
-
- WHO/PAHO/CDC. Measles Eradication: Recommendations from a Meeting Cosponsored by the World Health Organization, the Pan American Health Organization, and CDC. CDC-MMWR [Internet]. 1997;46(RR11):1–20. Available: http://www.cdc.gov/mmwr/preview/mmwrhtml/00047959.htm - PubMed
-
- Helfand RF, Heath JL, Anderson LJ, Maes EF, Guris D, Bellini WJ. Diagnosis of measles with an IgM capture EIA: the optimal timing of specimen collection after rash onset. J Infect Dis [Internet]. 1997. [cited 2013 Nov 2];175(1):195–9. Available: http://jid.oxfordjournals.org/content/175/1/195.short - PubMed
-
- WHO. Measles Surveillance Data [Internet]. Immunization, Vaccines and Biologicals. 2015 [cited 2015 Sep 5]. Available: http://www.who.int/immunization/monitoring_surveillance/burden/vpd/surve...
-
- WHO. Measles (Fact sheet N°286) [Internet]. Media centre. 2015 [cited 2015 Sep 5]. Available: http://www.who.int/mediacentre/factsheets/fs286/en/
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

