Toward Structural Correctness: Aquatolide and the Importance of 1D Proton NMR FID Archiving
- PMID: 26812443
- PMCID: PMC4746703
- DOI: 10.1021/acs.joc.5b02456
Toward Structural Correctness: Aquatolide and the Importance of 1D Proton NMR FID Archiving
Abstract
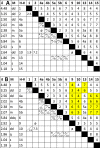
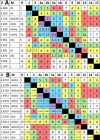
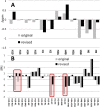
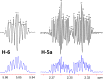
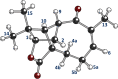
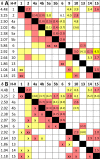
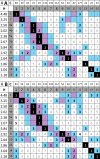
The revision of the structure of the sesquiterpene aquatolide from a bicyclo[2.2.0]hexane to a bicyclo[2.1.1]hexane structure using compelling NMR data, X-ray crystallography, and the recent confirmation via full synthesis exemplify that the achievement of "structural correctness" depends on the completeness of the experimental evidence. Archived FIDs and newly acquired aquatolide spectra demonstrate that archiving and rigorous interpretation of 1D (1)H NMR data may enhance the reproducibility of (bio)chemical research and curb the growing trend of structural misassignments. Despite being the most accessible NMR experiment, 1D (1)H spectra encode a wealth of information about bonds and molecular geometry that may be fully mined by (1)H iterative full spin analysis (HiFSA). Fully characterized 1D (1)H spectra are unideterminant for a given structure. The corresponding FIDs may be readily submitted with publications and collected in databases. Proton NMR spectra are indispensable for structural characterization even in conjunction with 2D data. Quantum interaction and linkage tables (QuILTs) are introduced for a more intuitive visualization of 1D J-coupling relationships, NOESY correlations, and heteronuclear experiments. Overall, this study represents a significant contribution to best practices in NMR-based structural analysis and dereplication.
Conflict of interest statement
M.N. is founder of Perch Solutions Limited. The other authors declare no competing financial interest.
Figures

References
-
- San Feliciano A.; Medarde M.; Miguel del Corral J. M.; Aramburu A.; Gordaliza M.; Barrero A. F. Tetrahedron Lett. 1989, 30, 2851–2854. 10.1016/S0040-4039(00)99142-1. - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

