Validation of a Multiplex Allele-Specific Polymerase Chain Reaction Assay for Detection of KRAS Gene Mutations in Formalin-Fixed, Paraffin-Embedded Tissues from Colorectal Cancer Patients
- PMID: 26812617
- PMCID: PMC4728071
- DOI: 10.1371/journal.pone.0147672
Validation of a Multiplex Allele-Specific Polymerase Chain Reaction Assay for Detection of KRAS Gene Mutations in Formalin-Fixed, Paraffin-Embedded Tissues from Colorectal Cancer Patients
Abstract
Background: Patients with KRAS mutations do not respond to epidermal growth factor receptor (EGFR) inhibitors and fail to benefit from adjuvant chemotherapy. Mutation analysis of KRAS is needed before starting treatment with monoclonal anti-EGFR antibodies in patients with metastatic colorectal cancer (mCRC). The objective of this study is to develop a multiplex allele-specific PCR (MAS-PCR) assay to detect KRAS mutations.
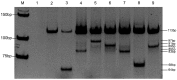
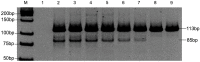
Methods: We developed a single-tube MAS-PCR assay for the detection of seven KRAS mutations (G12D, G12A, G12R, G12C, G12S, G12V, and G13D). We performed MAS-PCR assay analysis for KRAS on DNA isolated from 270 formalin-fixed paraffin-embedded (FFPE) colorectal cancer tissues. Sequences of all 270 samples were determined by pyrosequencing. Seven known point-mutation DNA samples diluted with wild-type DNA were assayed to determine the limitation of detection and reproducibility of the MAS-PCR assay.
Results: Overall, the results of MAS-PCR assay were in good concordance with pyrosequencing, and only seven discordant samples were found. The MAS-PCR assay reproducibly detected 1 to 2% mutant alleles. The most common mutations were G13D in codon 13 (49.17%), G12D (25.83%) and G12V (12.50%) in codon 12.
Conclusion: The MAS-PCR assay provides a rapid, cost-effective, and reliable diagnostic tool for accurate detection of KRAS mutations in routine FFPE colorectal cancer tissues.
Conflict of interest statement
Figures

Similar articles
-
Optimized Multiplex Detection of 7 KRAS Mutations by Taqman Allele-Specific qPCR.PLoS One. 2016 Sep 15;11(9):e0163070. doi: 10.1371/journal.pone.0163070. eCollection 2016. PLoS One. 2016. PMID: 27632281 Free PMC article.
-
KRas and BRaf mutational status analysis from formalin-fixed, paraffin-embedded tissues using multiplex polymerase chain reaction-based assay.Arch Pathol Lab Med. 2010 Apr;134(4):620-4. doi: 10.5858/134.4.620. Arch Pathol Lab Med. 2010. PMID: 20367313
-
Detection of KRAS codon 12 and 13 mutations by mutant-enriched PCR assay.Clin Chim Acta. 2014 Sep 25;436:169-75. doi: 10.1016/j.cca.2014.05.008. Epub 2014 May 24. Clin Chim Acta. 2014. PMID: 24863805
-
Sensitive multiplex detection of KRAS codons 12 and 13 mutations in paraffin-embedded tissue specimens.J Clin Pathol. 2011 Jan;64(1):30-6. doi: 10.1136/jcp.2010.081539. Epub 2010 Oct 28. J Clin Pathol. 2011. PMID: 21030527
-
KRAS and BRAF mutation analysis in colorectal adenocarcinoma specimens with a low percentage of tumor cells.Mol Diagn Ther. 2013 Jun;17(3):193-203. doi: 10.1007/s40291-013-0025-8. Mol Diagn Ther. 2013. PMID: 23606169 Free PMC article.
Cited by
-
Single-copy detection of somatic variants from solid and liquid biopsy.Sci Rep. 2021 Mar 16;11(1):6068. doi: 10.1038/s41598-021-85545-3. Sci Rep. 2021. PMID: 33727644 Free PMC article.
-
Development of ultra-short PCR assay to reveal BRAF V600 mutation status in Thai colorectal cancer tissues.PLoS One. 2018 Jun 7;13(6):e0198795. doi: 10.1371/journal.pone.0198795. eCollection 2018. PLoS One. 2018. PMID: 29879227 Free PMC article.
-
Miniaturized Real-Time PCR on a Q3 System for Rapid KRAS Genotyping.Sensors (Basel). 2017 Apr 11;17(4):831. doi: 10.3390/s17040831. Sensors (Basel). 2017. PMID: 28398227 Free PMC article.
-
Metabolic characterization of colorectal cancer cells harbouring different KRAS mutations in codon 12, 13, 61 and 146 using human SW48 isogenic cell lines.Metabolomics. 2020 Apr 16;16(4):51. doi: 10.1007/s11306-020-01674-2. Metabolomics. 2020. PMID: 32300895 Free PMC article.
-
Optimized Multiplex Detection of 7 KRAS Mutations by Taqman Allele-Specific qPCR.PLoS One. 2016 Sep 15;11(9):e0163070. doi: 10.1371/journal.pone.0163070. eCollection 2016. PLoS One. 2016. PMID: 27632281 Free PMC article.
References
-
- Sriamporn S, Wiangnon S, Suwanrungruang K, Rungsrikaji D, Sukprasert A, Thipsuntornsak N, et al. Risk factors for colorectal cancer in northeast Thailand: lifestyle related. Asian Pac J Cancer Prev. 2007;8(4): 573–577. - PubMed
-
- Sangrajrang S, Chokvanitphong V, Sumetchotimaytha W, Khuhaprema T. Evaluation of health status of a population underwent routine medical check up at the high risk screening clinic in National Cancer Institute. Asian Pac J Cancer Prev. 2012;13(11): 5759–5762. - PubMed
-
- Bosman F, Yan P. Molecular pathology of colorectal cancer. Pol J Pathol. 2014;65(4): 257–266. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

