Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression
- PMID: 26812922
- PMCID: PMC4728392
- DOI: 10.1038/srep19781
Skeletal muscle Heat shock protein 60 increases after endurance training and induces peroxisome proliferator-activated receptor gamma coactivator 1 α1 expression
Abstract
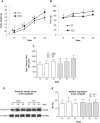
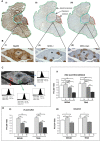
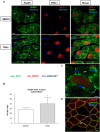
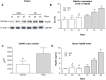
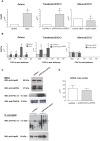
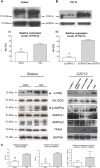
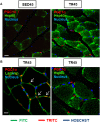
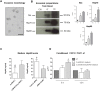
Heat shock protein 60 (Hsp60) is a chaperone localizing in skeletal muscle mitochondria, whose role is poorly understood. In the present study, the levels of Hsp60 in fibres of the entire posterior group of hindlimb muscles (gastrocnemius, soleus, and plantaris) were evaluated in mice after completing a 6-week endurance training program. The correlation between Hsp60 levels and the expression of four isoforms of peroxisome proliferator-activated receptor gamma coactivator 1 alpha (PGC1α) were investigated only in soleus. Short-term overexpression of hsp60, achieved by in vitro plasmid transfection, was then performed to determine whether this chaperone could have a role in the activation of the expression levels of PGC1α isoforms. The levels of Hsp60 protein were fibre-type specific in the posterior muscles and endurance training increased its content in type I muscle fibers. Concomitantly with the increased levels of Hsp60 released in the blood stream of trained mice, mitochondrial copy number and the expression of three isoforms of PGC1α increased. Overexpressing hsp60 in cultured myoblasts induced only the expression of PGC1 1α, suggesting a correlation between Hsp60 overexpression and PGC1 1 α activation.
Figures
References
-
- Czarnecka A. M., Campanella C., Zummo G. & Cappello F. Mitochondrial chaperones in cancer: from molecular biology to clinical diagnostics. Cancer biology & therapy 5, 714–720 (2006). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

