Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial
- PMID: 26813210
- PMCID: PMC4824537
- DOI: 10.1001/jama.2015.19284
Effects of Nicotine Patch vs Varenicline vs Combination Nicotine Replacement Therapy on Smoking Cessation at 26 Weeks: A Randomized Clinical Trial
Abstract
Importance: Smoking cessation medications are routinely used in health care; it is vital to identify medications that most effectively treat this leading cause of preventable mortality.
Objective: To compare the efficacies of varenicline, combination nicotine replacement therapy (C-NRT), and the nicotine patch for 26-week quit rates.
Design, setting, and participants: Three-group randomized intention-to-treat clinical trial occurring from May 2012 to November 2015 among smokers recruited in the Madison, Wisconsin, and Milwaukee, Wisconsin, communities; 65.5% of smokers offered the study (2687/4102) refused participation prior to randomization.
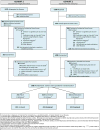
Interventions: Participants were randomized to one of three 12-week open-label smoking cessation pharmacotherapy groups: (1) nicotine patch only (n = 241); (2) varenicline only (including 1 prequit week; n = 424); and (3) C-NRT (nicotine patch + nicotine lozenge; n = 421). Six counseling sessions were offered.
Main outcomes and measures: The primary outcome was carbon monoxide-confirmed self-reported 7-day point-prevalence abstinence at 26 weeks. Secondary outcomes were carbon monoxide-confirmed self-reported initial abstinence, prolonged abstinence at 26 weeks, and point-prevalence abstinence at weeks 4, 12, and 52.
Results: Among 1086 smokers randomized (52% women; 67% white; mean age, 48 years; mean of 17 cigarettes smoked per day), 917 (84%) provided 12-month follow-up data. Treatments did not differ on any abstinence outcome measure at 26 or 52 weeks, including point-prevalence abstinence at 26 weeks (nicotine patch, 22.8% [55/241]; varenicline, 23.6% [100/424]; and C-NRT, 26.8% [113/421]) or at 52 weeks (nicotine patch, 20.8% [50/241]; varenicline, 19.1% [81/424]; and C-NRT, 20.2% [85/421]). At 26 weeks, the risk differences for abstinence were, for patch vs varenicline, -0.76% (95% CI, -7.4% to 5.9%); for patch vs C-NRT, -4.0% (95% CI, -10.8% to 2.8%); and for varenicline vs C-NRT, -3.3% (95% CI, -9.1% to 2.6%). All medications were well tolerated, but varenicline produced more frequent adverse events than did the nicotine patch for vivid dreams, insomnia, nausea, constipation, sleepiness, and indigestion.
Conclusions and relevance: Among adults motivated to quit smoking, 12 weeks of open-label treatment with nicotine patch, varenicline, or C-NRT produced no significant differences in biochemically confirmed rates of smoking abstinence at 26 weeks. The results raise questions about the relative effectiveness of intense smoking pharmacotherapies.
Trial registration: clinicaltrials.gov Identifier: NCT01553084.
Conflict of interest statement
Timothy Baker reports no conflict of interest. Megan Piper reports no conflict of interest. James Stein reports no conflict of interest. Stevens Smith reports no conflict of interest. Dan Bolt reports no conflict of interest. David Fraser reports no conflict of interest. Michael Fiore reports no conflict of interest.
Comment in
-
Similar quit rates are found with three smoking cessation options.BMJ. 2016 Jan 26;352:i449. doi: 10.1136/bmj.i449. BMJ. 2016. PMID: 26818201 No abstract available.
-
Varenicline, combination NRT, and nicotine patch did not differ for smoking cessation at 6 mo.Ann Intern Med. 2016 May 17;164(10):JC54. doi: 10.7326/ACPJC-2016-164-10-054. Ann Intern Med. 2016. PMID: 27182920 No abstract available.
-
La nicotine aussi efficace que la varénicline?Rev Med Suisse. 2016 Mar 23;12(511):620. Rev Med Suisse. 2016. PMID: 27188059 French. No abstract available.
Similar articles
-
Flexible, dual-form nicotine replacement therapy or varenicline in comparison with nicotine patch for smoking cessation: a randomized controlled trial.BMC Med. 2016 Jun 7;14:80. doi: 10.1186/s12916-016-0626-2. BMC Med. 2016. PMID: 27233840 Free PMC article. Clinical Trial.
-
Efficacy of varenicline combined with nicotine replacement therapy vs varenicline alone for smoking cessation: a randomized clinical trial.JAMA. 2014 Jul;312(2):155-61. doi: 10.1001/jama.2014.7195. JAMA. 2014. PMID: 25005652 Clinical Trial.
-
An exploratory short-term double-blind randomized trial of varenicline versus nicotine patch for smoking cessation in women.Addiction. 2015 Jun;110(6):1027-34. doi: 10.1111/add.12895. Epub 2015 Mar 29. Addiction. 2015. PMID: 25727442 Free PMC article. Clinical Trial.
-
Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2019 Apr 18;4(4):CD013308. doi: 10.1002/14651858.CD013308. Cochrane Database Syst Rev. 2019. Update in: Cochrane Database Syst Rev. 2023 Jun 19;6:CD013308. doi: 10.1002/14651858.CD013308.pub2. PMID: 30997928 Free PMC article. Updated.
-
A review of the efficacy of smoking-cessation pharmacotherapies in nonwhite populations.Clin Ther. 2008 May;30(5):800-12. doi: 10.1016/j.clinthera.2008.05.010. Clin Ther. 2008. PMID: 18555928 Review.
Cited by
-
The effect of area-level disadvantage and race on smoking abstinence in a clinical trial.Exp Clin Psychopharmacol. 2022 Jun;30(3):279-286. doi: 10.1037/pha0000493. Epub 2021 Aug 9. Exp Clin Psychopharmacol. 2022. PMID: 34370500 Free PMC article. Clinical Trial.
-
Moderators of smoking cessation outcomes in a randomized-controlled trial of varenicline versus placebo.Psychopharmacology (Berl). 2017 Dec;234(23-24):3417-3429. doi: 10.1007/s00213-017-4721-7. Epub 2017 Sep 9. Psychopharmacology (Berl). 2017. PMID: 28889258 Clinical Trial.
-
Effects of Negative Affect, Urge to Smoke, and Working Memory Performance (n-back) on Nicotine Dependence.Subst Use Misuse. 2018 Jun 7;53(7):1177-1183. doi: 10.1080/10826084.2017.1400569. Epub 2017 Nov 29. Subst Use Misuse. 2018. PMID: 29185837 Free PMC article.
-
Psychological features of adult patients with langerhans cell histiocytosis.PLoS One. 2021 Feb 12;16(2):e0246604. doi: 10.1371/journal.pone.0246604. eCollection 2021. PLoS One. 2021. PMID: 33577606 Free PMC article.
-
Different doses, durations and modes of delivery of nicotine replacement therapy for smoking cessation.Cochrane Database Syst Rev. 2023 Jun 19;6(6):CD013308. doi: 10.1002/14651858.CD013308.pub2. Cochrane Database Syst Rev. 2023. PMID: 37335995 Free PMC article.
References
-
- Centers for Disease Control and Prevention. Smoking-attributable mortality, years of potential life lost, and productivity losses–United States, 2000–2004. MMWR Morb Mortal Wkly Rep. 2008;57(45):1226–1228. - PubMed
-
- Fiore MC, Jaen CR, Baker TB, et al. Treating tobacco use and dependence: 2008 update. Rockville, MD: U.S. Department of Health and Human Services, U.S. Public Health Service; 2008.
-
- Hsueh KC, Hsueh SC, Chou MY, et al. Varenicline versus transdermal nicotine patch: a 3-year follow-up in a smoking cessation clinic in Taiwan. Psychopharmacology (Berl) 2014;231(14):2819–2823. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical