Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming
- PMID: 26813411
- PMCID: PMC4729172
- DOI: 10.1186/s13071-016-1314-y
Multi-parallel qPCR provides increased sensitivity and diagnostic breadth for gastrointestinal parasites of humans: field-based inferences on the impact of mass deworming
Abstract
Background: Although chronic morbidity in humans from soil transmitted helminth (STH) infections can be reduced by anthelmintic treatment, inconsistent diagnostic tools make it difficult to reliably measure the impact of deworming programs and often miss light helminth infections.
Methods: Cryopreserved stool samples from 796 people (aged 2-81 years) in four villages in Bungoma County, western Kenya, were assessed using multi-parallel qPCR for 8 parasites and compared to point-of-contact assessments of the same stools by the 2-stool 2-slide Kato-Katz (KK) method. All subjects were treated with albendazole and all Ascaris lumbricoides expelled post-treatment were collected. Three months later, samples from 633 of these people were re-assessed by both qPCR and KK, re-treated with albendazole and the expelled worms collected.
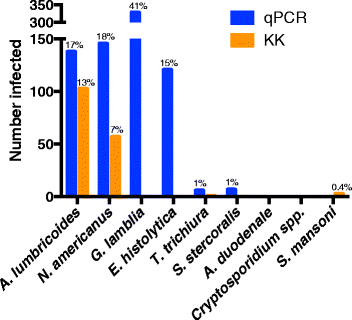
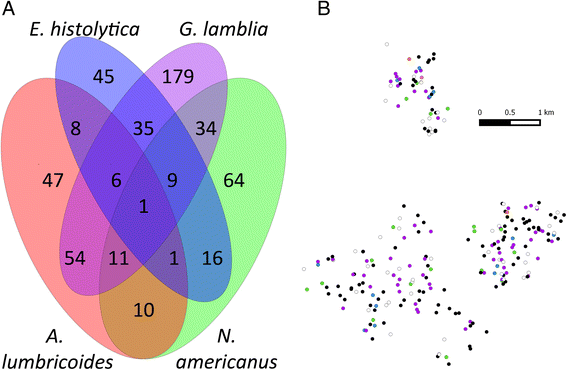
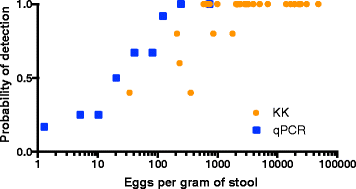
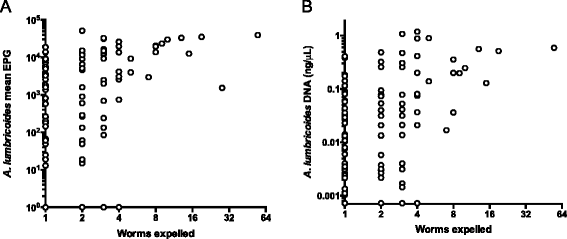
Results: Baseline prevalence by qPCR (n = 796) was 17 % for A. lumbricoides, 18 % for Necator americanus, 41 % for Giardia lamblia and 15% for Entamoeba histolytica. The prevalence was <1% for Trichuris trichiura, Ancylostoma duodenale, Strongyloides stercoralis and Cryptosporidium parvum. The sensitivity of qPCR was 98% for A. lumbricoides and N. americanus, whereas KK sensitivity was 70% and 32%, respectively. Furthermore, qPCR detected infections with T. trichiura and S. stercoralis that were missed by KK, and infections with G. lamblia and E. histolytica that cannot be detected by KK. Infection intensities measured by qPCR and by KK were correlated for A. lumbricoides (r = 0.83, p < 0.0001) and N. americanus (r = 0.55, p < 0.0001). The number of A. lumbricoides worms expelled was correlated (p < 0.0001) with both the KK (r = 0.63) and qPCR intensity measurements (r = 0.60).
Conclusions: KK may be an inadequate tool for stool-based surveillance in areas where hookworm or Strongyloides are common or where intensity of helminth infection is low after repeated rounds of chemotherapy. Because deworming programs need to distinguish between populations where parasitic infection is controlled and those where further treatment is required, multi-parallel qPCR (or similar high throughput molecular diagnostics) may provide new and important diagnostic information.
Figures


References
-
- WHO . Soil-transmitted helminthiases: number of children treated in 2013: World Health Organization. 2015. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

