Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury
- PMID: 26813496
- PMCID: PMC4737895
- DOI: 10.1038/ncomms10523
Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury
Erratum in
-
Publisher Correction: Rapid endothelial cytoskeletal reorganization enables early blood-brain barrier disruption and long-term ischaemic reperfusion brain injury.Nat Commun. 2020 Aug 25;11(1):4335. doi: 10.1038/s41467-020-18263-5. Nat Commun. 2020. PMID: 32843701 Free PMC article.
Abstract
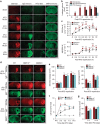
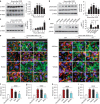
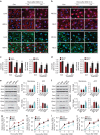
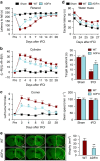
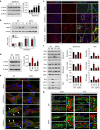
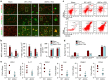
The mechanism and long-term consequences of early blood-brain barrier (BBB) disruption after cerebral ischaemic/reperfusion (I/R) injury are poorly understood. Here we discover that I/R induces subtle BBB leakage within 30-60 min, likely independent of gelatinase B/MMP-9 activities. The early BBB disruption is caused by the activation of ROCK/MLC signalling, persistent actin polymerization and the disassembly of junctional proteins within microvascular endothelial cells (ECs). Furthermore, the EC alterations facilitate subsequent infiltration of peripheral immune cells, including MMP-9-producing neutrophils/macrophages, resulting in late-onset, irreversible BBB damage. Inactivation of actin depolymerizing factor (ADF) causes sustained actin polymerization in ECs, whereas EC-targeted overexpression of constitutively active mutant ADF reduces actin polymerization and junctional protein disassembly, attenuates both early- and late-onset BBB impairment, and improves long-term histological and neurological outcomes. Thus, we identify a previously unexplored role for early BBB disruption in stroke outcomes, whereby BBB rupture may be a cause rather than a consequence of parenchymal cell injury.
Figures




References
-
- Khatri R., McKinney A. M., Swenson B. & Janardhan V. Blood-brain barrier, reperfusion injury, and hemorrhagic transformation in acute stroke. Neurology 79, S52–S57 (2012). - PubMed
-
- Neumann-Haefelin T. et al.. Serial MRI after transient focal cerebral ischemia in rats: dynamics of tissue injury, blood-brain barrier damage, and edema formation. Stroke 31, 1965–1972 (2000). - PubMed
-
- Olah L., Wecker S. & Hoehn M. Secondary deterioration of apparent diffusion coefficient after 1-hour transient focal cerebral ischemia in rats. J. Cereb. Blood Flow Metab. 20, 1474–1482 (2000). - PubMed
-
- Kaur J. et al.. Quantified T1 as an adjunct to apparent diffusion coefficient for early infarct detection: a high-field magnetic resonance study in a rat stroke model. Int. J. Stroke 4, 159–168 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

