Regulation of human cerebro-microvascular endothelial baso-lateral adhesion and barrier function by S1P through dual involvement of S1P1 and S1P2 receptors
- PMID: 26813587
- PMCID: PMC4728386
- DOI: 10.1038/srep19814
Regulation of human cerebro-microvascular endothelial baso-lateral adhesion and barrier function by S1P through dual involvement of S1P1 and S1P2 receptors
Abstract
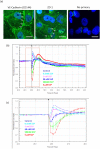
Herein we show that S1P rapidly and acutely reduces the focal adhesion strength and barrier tightness of brain endothelial cells. xCELLigence biosensor technology was used to measure focal adhesion, which was reduced by S1P acutely and this response was mediated through both S1P1 and S1P2 receptors. S1P increased secretion of several pro-inflammatory mediators from brain endothelial cells. However, the magnitude of this response was small in comparison to that mediated by TNFα or IL-1β. Furthermore, S1P did not significantly increase cell-surface expression of any key cell adhesion molecules involved in leukocyte recruitment, included ICAM-1 and VCAM-1. Finally, we reveal that S1P acutely and dynamically regulates microvascular endothelial barrier tightness in a manner consistent with regulated rapid opening followed by closing and strengthening of the barrier. We hypothesise that the role of the S1P receptors in this process is not to cause barrier dysfunction, but is related to controlled opening of the endothelial junctions. This was revealed using real-time measurement of barrier integrity using ECIS ZΘ TEER technology and endothelial viability using xCELLigence technology. Finally, we show that these responses do not occur simply though the pharmacology of a single S1P receptor but involves coordinated action of S1P1 and S1P2 receptors.
Figures






References
-
- Brinkmann V. sphingosine 1-phosphate receptors in health and disease. Mechanistic insights from gene deletion studies and reverse pharmacology. pharmacology and therapeutics 115, 84–105 (2007). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

