The Opposing Actions of Arabidopsis CHROMOSOME TRANSMISSION FIDELITY7 and WINGS APART-LIKE1 and 2 Differ in Mitotic and Meiotic Cells
- PMID: 26813623
- PMCID: PMC4790872
- DOI: 10.1105/tpc.15.00781
The Opposing Actions of Arabidopsis CHROMOSOME TRANSMISSION FIDELITY7 and WINGS APART-LIKE1 and 2 Differ in Mitotic and Meiotic Cells
Abstract
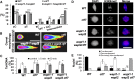
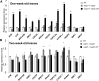
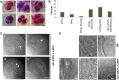
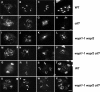
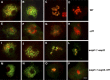
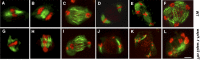
Sister chromatid cohesion, which is mediated by the cohesin complex, is essential for the proper segregation of chromosomes during mitosis and meiosis. Stable binding of cohesin with chromosomes is regulated in part by the opposing actions of CTF7 (CHROMOSOME TRANSMISSION FIDELITY7) and WAPL (WINGS APART-LIKE). In this study, we characterized the interaction between Arabidopsis thaliana CTF7 and WAPL by conducting a detailed analysis of wapl1-1 wapl2 ctf7 plants. ctf7 plants exhibit major defects in vegetative growth and development and are completely sterile. Inactivation of WAPL restores normal growth, mitosis, and some fertility to ctf7 plants. This shows that the CTF7/WAPL cohesin system is not essential for mitosis in vegetative cells and suggests that plants may contain a second mechanism to regulate mitotic cohesin. WAPL inactivation restores cohesin binding and suppresses ctf7-associated meiotic cohesion defects, demonstrating that WAPL and CTF7 function as antagonists to regulate meiotic sister chromatid cohesion. The ctf7 mutation only had a minor effect on wapl-associated defects in chromosome condensation and centromere association. These results demonstrate that WAPL has additional roles that are independent of its role in regulating chromatin-bound cohesin.
© 2016 American Society of Plant Biologists. All rights reserved.
Figures


Similar articles
-
The Role of Structural Maintenance of Chromosomes Complexes in Meiosis and Genome Maintenance: Translating Biomedical and Model Plant Research Into Crop Breeding Opportunities.Front Plant Sci. 2021 Mar 31;12:659558. doi: 10.3389/fpls.2021.659558. eCollection 2021. Front Plant Sci. 2021. PMID: 33868354 Free PMC article. Review.
-
Arabidopsis thaliana WAPL is essential for the prophase removal of cohesin during meiosis.PLoS Genet. 2014 Jul 17;10(7):e1004497. doi: 10.1371/journal.pgen.1004497. eCollection 2014 Jul. PLoS Genet. 2014. PMID: 25033056 Free PMC article.
-
AtCTF7 is required for establishment of sister chromatid cohesion and association of cohesin with chromatin during meiosis in Arabidopsis.BMC Plant Biol. 2013 Aug 14;13:117. doi: 10.1186/1471-2229-13-117. BMC Plant Biol. 2013. PMID: 23941555 Free PMC article.
-
Arabidopsis CHROMOSOME TRANSMISSION FIDELITY 7 (AtCTF7/ECO1) is required for DNA repair, mitosis and meiosis.Plant J. 2013 Sep;75(6):927-40. doi: 10.1111/tpj.12261. Epub 2013 Jul 20. Plant J. 2013. PMID: 23750584 Free PMC article.
-
Centromeric Cohesin: Molecular Glue and Much More.Prog Mol Subcell Biol. 2017;56:485-513. doi: 10.1007/978-3-319-58592-5_20. Prog Mol Subcell Biol. 2017. PMID: 28840250 Review.
Cited by
-
The Role of Structural Maintenance of Chromosomes Complexes in Meiosis and Genome Maintenance: Translating Biomedical and Model Plant Research Into Crop Breeding Opportunities.Front Plant Sci. 2021 Mar 31;12:659558. doi: 10.3389/fpls.2021.659558. eCollection 2021. Front Plant Sci. 2021. PMID: 33868354 Free PMC article. Review.
-
Sororin is an evolutionary conserved antagonist of WAPL.Nat Commun. 2024 Jun 3;15(1):4729. doi: 10.1038/s41467-024-49178-0. Nat Commun. 2024. PMID: 38830897 Free PMC article.
-
Histone H2A monoubiquitination marks are targeted to specific sites by cohesin subunits in Arabidopsis.Nat Commun. 2023 Mar 3;14(1):1209. doi: 10.1038/s41467-023-36788-3. Nat Commun. 2023. PMID: 36869051 Free PMC article.
-
In Favor of Establishment: Regulation of Chromatid Cohesion in Plants.Front Plant Sci. 2017 May 23;8:846. doi: 10.3389/fpls.2017.00846. eCollection 2017. Front Plant Sci. 2017. PMID: 28588601 Free PMC article. Review.
References
-
- Armstrong S.J., Caryl A.P., Jones G.H., Franklin F.C. (2002). Asy1, a protein required for meiotic chromosome synapsis, localizes to axis-associated chromatin in Arabidopsis and Brassica. J. Cell Sci. 115: 3645–3655. - PubMed
-
- Arumugam P., Nishino T., Haering C.H., Gruber S., Nasmyth K. (2006). Cohesin’s ATPase activity is stimulated by the C-terminal Winged-Helix domain of its kleisin subunit. Curr. Biol. 16: 1998–2008. - PubMed
-
- Bennett C.B., Lewis L.K., Karthikeyan G., Lobachev K.S., Jin Y.H., Sterling J.F., Snipe J.R., Resnick M.A. (2001). Genes required for ionizing radiation resistance in yeast. Nat. Genet. 29: 426–434. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

