Published and unpublished evidence in coverage decision-making for pharmaceuticals in Europe: existing approaches and way forward
- PMID: 26813738
- PMCID: PMC4727332
- DOI: 10.1186/s12961-016-0080-9
Published and unpublished evidence in coverage decision-making for pharmaceuticals in Europe: existing approaches and way forward
Abstract
Background: Dissemination bias occurs when only some results emerging from clinical research reach their intended audience in the knowledge translation process. Given that coverage decisions increasingly rely on evidence, it is important to explore the types of evidence considered. This paper aimed to examine the evidence base used by regulatory institutions involved in pricing and reimbursement of pharmaceuticals in a broad range of European countries, as well as their awareness of and approach towards dissemination bias.
Methods: A mixed methods approach was adopted. Regulatory documents and published literature were identified in systematic searches and relevant documents were analysed. An online survey was carried out to verify and expand insights.
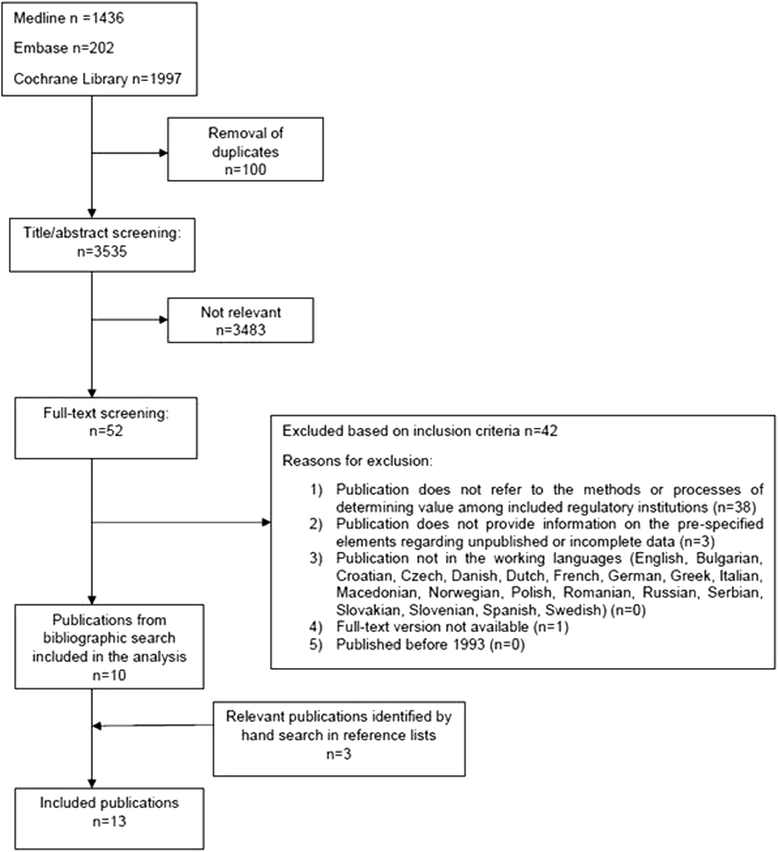
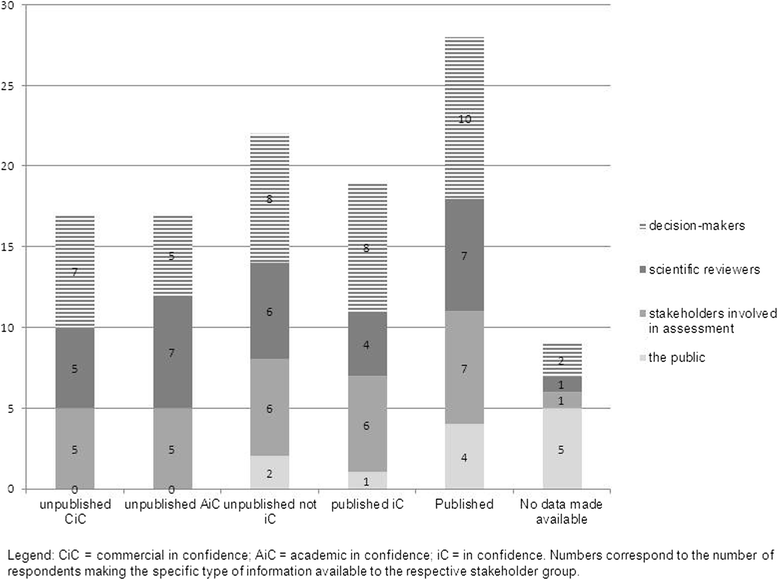
Results: Forty-two relevant regulatory documents and 10 publications were included. The survey had a 35% response rate, yielding valid responses for 13 countries. A fragmented impression was obtained for most countries indicating a general lack of transparency regarding both processes of decision-making and approaches towards unpublished information. Dissemination bias was rarely consistently considered. Practices for the identification and inclusion of all available evidence varied considerably, as did the influence of missing evidence on decision-making. Differences were often attributable to the regulatory context and/or institutional principles.
Conclusions: Best practice is difficult to generalize given the identified variations. Individual exemplary practices support the necessity for institutional exchange at international level. Increased institutional commitment to transparency of methods and processes should be advocated.
Figures

References
-
- Song F, Eastwood AJ, Gilbody S, Duley L, Sutton AJ. Publication and related biases. Health Technol Assess. 2000;4:1–115. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

