Rigid Adenine Nucleoside Derivatives as Novel Modulators of the Human Sodium Symporters for Dopamine and Norepinephrine
- PMID: 26813929
- PMCID: PMC4809312
- DOI: 10.1124/jpet.115.229666
Rigid Adenine Nucleoside Derivatives as Novel Modulators of the Human Sodium Symporters for Dopamine and Norepinephrine
Abstract
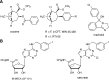
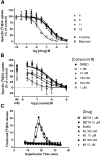
Thirty-two congeneric rigid adenine nucleoside derivatives containing a North (N)-methanocarba ribose substitution and a 2-arylethynyl group either enhanced (up to 760% of control) or inhibited [(125)I] methyl (1R,2S,3S)-3-(4-iodophenyl)-8-methyl-8-azabicyclo[3.2.1]octane-2-carboxylate (RTI-55) binding at the human dopamine (DA) transporter (DAT) and inhibited DA uptake. Several nucleosides also enhanced [(3)H]mazindol [(±)-5-(4-chlorophenyl)-3,5-dihydro-2H-imidazo[2,1-a]isoindol-5-ol] binding to the DAT. The combination of binding enhancement and functional inhibition suggests possible allosteric interaction with the tropanes. The structure-activity relationship of this novel class of DAT ligands was explored: small N(6)-substition (methyl or ethyl) was favored, while the N1 of the adenine ring was essential. Effective terminal aryl groups include thien-2-yl (compounds 9 and 16), with EC50 values of 35.1 and 9.1 nM, respectively, in [(125)I]RTI-55 binding enhancement, and 3,4-difluorophenyl as in the most potent DA uptake inhibitor (compound 6) with an IC50 value of 92 nM (3-fold more potent than cocaine), but not nitrogen heterocycles. Several compounds inhibited or enhanced binding at the norepinephrine transporter (NET) and serotonin transporter (SERT) and inhibited function in the micromolar range; truncation at the 4'-position in compound 23 allowed for weak inhibition of the SERT. We have not yet eliminated adenosine receptor affinity from this class of DAT modulators, but we identified modifications that remove DAT inhibition as an off-target effect of potent adenosine receptor agonists. Thus, we have identified a new class of allosteric DAT ligands, rigidified adenosine derivatives, and explored their initial structural requirements. They display a very atypical pharmacological profile, i.e., either enhancement by increasing affinity or inhibition of radioligand binding at the DAT, and in some cases the NET and SERT, and inhibition of neurotransmitter uptake.
U.S. Government work not protected by U.S. copyright.
Figures





References
-
- Axelrod J, Whitby LG, Hertting G. (1961) Effect of psychotropic drugs on the uptake of H3-norepinephrine by tissues. Science 133:383–384. - PubMed
-
- Borea PA, Varani K, Vincenzi F, Baraldi PG, Tabrizi MA, Merighi S, Gessi S. (2015) The A3 adenosine receptor: history and perspectives. Pharmacol Rev 67:74–102. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

