A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells
- PMID: 26813960
- PMCID: PMC4728441
- DOI: 10.1038/srep19772
A novel method for evaluating antibody-dependent cell-mediated cytotoxicity by flowcytometry using cryopreserved human peripheral blood mononuclear cells
Abstract
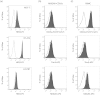
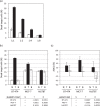
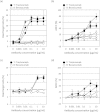
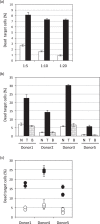
Analyzing the cytotoxic functions of effector cells, such as NK cells against target cancer cells, is thought to be necessary for predicting the clinical efficacy of antibody-dependent cellular cytotoxicity (ADCC) -dependent antibody therapy. The (51)Cr release assay has long been the most widely used method for quantification of ADCC activity. However, the reproducibilities of these release assays are not adequate, and they do not allow evaluation of the lysis susceptibilities of distinct cell types within the target cell population. In this study, we established a novel method for evaluating cytotoxicity, which involves the detection and quantification of dead target cells using flowcytometry. CFSE (carboxyfluorescein succinimidyl ester) was used as a dye to specifically stain and thereby label the target cell population, allowing living and dead cells, as well as both target and effector cells, to be quantitatively distinguished. Furthermore, with our new approach, ADCC activity was more reproducibly, sensitively, and specifically detectable, not only in freshly isolated but also in frozen human peripheral blood mononuclear cells (PBMCs), than with the calcein-AM release assay. This assay, validated herein, is expected to become a standard assay for evaluating ADCC activity which will ultimately contribute the clinical development of ADCC dependent-antibody therapies.
Figures

Similar articles
-
Thaw-and-use target cells pre-labeled with calcein AM for antibody-dependent cell-mediated cytotoxicity assays.J Immunol Methods. 2017 Aug;447:37-46. doi: 10.1016/j.jim.2017.04.005. Epub 2017 Apr 21. J Immunol Methods. 2017. PMID: 28434980
-
Quantitating ADCC against adherent cells: Impedance-based detection is superior to release, membrane permeability, or caspase activation assays in resolving antibody dose response.Cytometry A. 2017 Oct;91(10):1021-1029. doi: 10.1002/cyto.a.23247. Epub 2017 Sep 25. Cytometry A. 2017. PMID: 28945315
-
Role of monocytes in mediating HIV-specific antibody-dependent cellular cytotoxicity.J Immunol Methods. 2012 Oct 31;384(1-2):51-61. doi: 10.1016/j.jim.2012.07.006. Epub 2012 Jul 24. J Immunol Methods. 2012. PMID: 22841577
-
Effects of cryopreservation on effector cells for antibody dependent cell-mediated cytotoxicity (ADCC) and natural killer (NK) cell activity in (51)Cr-release and CD107a assays.J Immunol Methods. 2014 Apr;406:1-9. doi: 10.1016/j.jim.2014.01.017. Epub 2014 Feb 20. J Immunol Methods. 2014. PMID: 24561308 Free PMC article.
-
Cytokine release by single, immunophenotyped human cells: use of the reverse hemolytic plaque assay.Immunol Rev. 1991 Feb;119:23-39. doi: 10.1111/j.1600-065x.1991.tb00576.x. Immunol Rev. 1991. PMID: 2045121 Review. No abstract available.
Cited by
-
Past, Present, and Future of Rituximab-The World's First Oncology Monoclonal Antibody Therapy.Front Oncol. 2018 Jun 4;8:163. doi: 10.3389/fonc.2018.00163. eCollection 2018. Front Oncol. 2018. PMID: 29915719 Free PMC article. Review.
-
Development of a Macrophage-Based ADCC Assay.Vaccines (Basel). 2021 Jun 17;9(6):660. doi: 10.3390/vaccines9060660. Vaccines (Basel). 2021. PMID: 34204268 Free PMC article.
-
Immunoglobulin-like transcript 2 as an impaired anti-tumor cytotoxicity marker of natural killer cells in patients with hepatocellular carcinoma.Front Immunol. 2024 Apr 4;15:1389411. doi: 10.3389/fimmu.2024.1389411. eCollection 2024. Front Immunol. 2024. PMID: 38638429 Free PMC article.
-
Multiepitope supramolecular peptide nanofibers eliciting coordinated humoral and cellular antitumor immune responses.Sci Adv. 2022 Jul 22;8(29):eabm7833. doi: 10.1126/sciadv.abm7833. Epub 2022 Jul 20. Sci Adv. 2022. PMID: 35857833 Free PMC article.
-
Elotuzumab, a potential therapeutic humanized anti-SLAMF7 monoclonal antibody, enhances natural killer cell-mediated killing of primary effusion lymphoma cells.Cancer Immunol Immunother. 2022 Oct;71(10):2497-2509. doi: 10.1007/s00262-022-03177-6. Epub 2022 Mar 9. Cancer Immunol Immunother. 2022. PMID: 35262781 Free PMC article.
References
-
- Salinas-Jazmin N., Hisaki-Itaya E. & Velasco-Velazquez M. A. A flow cytometry-based assay for the evaluation of antibody-dependent cell-mediated cytotoxicity (ADCC) in cancer cells. Methods Mol Biol 1165, 241–52 (2014). - PubMed
-
- Lesterhuis W. J., Haanen J. B. & Punt C. J. Cancer immunotherapy–revisited. Nat Rev Drug Discov 10, 591–600 (2011). - PubMed
-
- Slamon D. J. et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science 244, 707–12 (1989). - PubMed
-
- Robinson A. G. et al. Molecular predictive factors in patients receiving trastuzumab-based chemotherapy for metastatic disease. Clin Breast Cancer 7, 254–61 (2006). - PubMed
-
- Slamon D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med 344, 783–92 (2001). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

