Proteome-wide analysis reveals widespread lysine acetylation of major protein complexes in the malaria parasite
- PMID: 26813983
- PMCID: PMC4728587
- DOI: 10.1038/srep19722
Proteome-wide analysis reveals widespread lysine acetylation of major protein complexes in the malaria parasite
Abstract
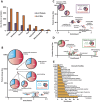
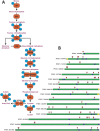
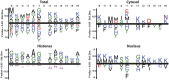
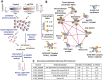
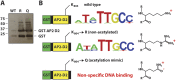
Lysine acetylation is a ubiquitous post-translational modification in many organisms including the malaria parasite Plasmodium falciparum, yet the full extent of acetylation across the parasite proteome remains unresolved. Moreover, the functional significance of acetylation or how specific acetyl-lysine sites are regulated is largely unknown. Here we report a seven-fold expansion of the known parasite 'acetylome', characterizing 2,876 acetylation sites on 1,146 proteins. We observe that lysine acetylation targets a diverse range of protein complexes and is particularly enriched within the Apicomplexan AP2 (ApiAP2) DNA-binding protein family. Using quantitative proteomics we determined that artificial perturbation of the acetate/acetyl-CoA balance alters the acetyl-lysine occupancy of several ApiAP2 DNA-binding proteins and related transcriptional proteins. This metabolic signaling could mediate significant downstream transcriptional responses, as we show that acetylation of an ApiAP2 DNA-binding domain ablates its DNA-binding propensity. Lastly, we investigated the acetyl-lysine targets of each class of lysine deacetylase in order to begin to explore how each class of enzyme contributes to regulating the P. falciparum acetylome.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

