Precision Medicine: Genetic Repair of Retinitis Pigmentosa in Patient-Derived Stem Cells
- PMID: 26814166
- PMCID: PMC4728485
- DOI: 10.1038/srep19969
Precision Medicine: Genetic Repair of Retinitis Pigmentosa in Patient-Derived Stem Cells
Abstract
Induced pluripotent stem cells (iPSCs) generated from patient fibroblasts could potentially be used as a source of autologous cells for transplantation in retinal disease. Patient-derived iPSCs, however, would still harbor disease-causing mutations. To generate healthy patient-derived cells, mutations might be repaired with new gene-editing technology based on the bacterial system of clustered regularly interspersed short palindromic repeats (CRISPR)/Cas9, thereby yielding grafts that require no patient immunosuppression. We tested whether CRISPR/Cas9 could be used in patient-specific iPSCs to precisely repair an RPGR point mutation that causes X-linked retinitis pigmentosa (XLRP). Fibroblasts cultured from a skin-punch biopsy of an XLRP patient were transduced to produce iPSCs carrying the patient's c.3070G > T mutation. The iPSCs were transduced with CRISPR guide RNAs, Cas9 endonuclease, and a donor homology template. Despite the gene's repetitive and GC-rich sequences, 13% of RPGR gene copies showed mutation correction and conversion to the wild-type allele. This is the first report using CRISPR to correct a pathogenic mutation in iPSCs derived from a patient with photoreceptor degeneration. This important proof-of-concept finding supports the development of personalized iPSC-based transplantation therapies for retinal disease.
Figures


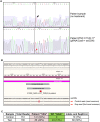
References
-
- Schwartz S. D. et al. Human embryonic stem cell-derived retinal pigment epithelium in patients with age-related macular degeneration and Stargardt’s macular dystrophy: follow-up of two open-label phase 1/2 studies. Lancet. 385, 509–516 (2015). - PubMed
-
- Takahashi K. et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 131, 861–872 (2007). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01EY018213/EY/NEI NIH HHS/United States
- R21 AG050437/AG/NIA NIH HHS/United States
- R01EY024698/EY/NEI NIH HHS/United States
- R01EY024665/EY/NEI NIH HHS/United States
- R01 EY018213/EY/NEI NIH HHS/United States
- 5P30EY019007/EY/NEI NIH HHS/United States
- R01 EY024665/EY/NEI NIH HHS/United States
- K08 EY020530/EY/NEI NIH HHS/United States
- 5P30CA013696/CA/NCI NIH HHS/United States
- K08EY020530/EY/NEI NIH HHS/United States
- R01 EY024698/EY/NEI NIH HHS/United States
- P30 EY019007/EY/NEI NIH HHS/United States
- R01 EY025225/EY/NEI NIH HHS/United States
- P30 CA013696/CA/NCI NIH HHS/United States
- R01EY025225/EY/NEI NIH HHS/United States
- R21AG050437/AG/NIA NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

