Cytocompatibility and biocompatibility of nanostructured carbonated hydroxyapatite spheres for bone repair
- PMID: 26814461
- PMCID: PMC4716697
- DOI: 10.1590/1678-775720150122
Cytocompatibility and biocompatibility of nanostructured carbonated hydroxyapatite spheres for bone repair
Abstract
Objective: The aim of this study was to investigate the in vitro and in vivo biological responses to nanostructured carbonated hydroxyapatite/calcium alginate (CHA) microspheres used for alveolar bone repair, compared to sintered hydroxyapatite (HA).
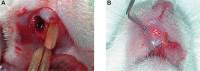
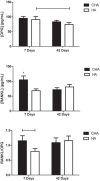
Material and methods: The maxillary central incisors of 45 Wistar rats were extracted, and the dental sockets were filled with HA, CHA, and blood clot (control group) (n=5/period/group). After 7, 21 and 42 days, the samples of bone with the biomaterials were obtained for histological and histomorphometric analysis, and the plasma levels of RANKL and OPG were determined via immunoassay. Statistical analysis was performed by Two-Way ANOVA with post-hoc Tukey test at 95% level of significance.
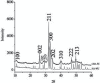
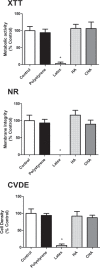
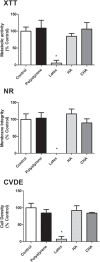
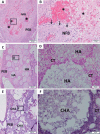
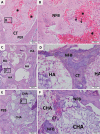
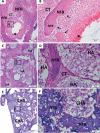
Results: The CHA and HA microspheres were cytocompatible with both human and murine cells on an in vitro assay. Histological analysis showed the time-dependent increase of newly formed bone in control group characterized by an intense osteoblast activity. In HA and CHA groups, the presence of a slight granulation reaction around the spheres was observed after seven days, which was reduced by the 42nd day. A considerable amount of newly formed bone was observed surrounding the CHA spheres and the biomaterials particles at 42-day time point compared with HA. Histomorphometric analysis showed a significant increase of newly formed bone in CHA group compared with HA after 21 and 42 days from surgery, moreover, CHA showed almost 2-fold greater biosorption than HA at 42 days (two-way ANOVA, p<0.05) indicating greater biosorption. An increase in the RANKL/OPG ratio was observed in the CHA group on the 7th day.
Conclusion: CHA spheres were osteoconductive and presented earlier biosorption, inducing early increases in the levels of proteins involved in resorption.
Figures


References
-
- Almasri M, Altalibi M. Efficacy of reconstruction of alveolar bone using an alloplastic hydroxyapatite tricalcium phosphate graft under biodegradable chambers. Br J Oral Maxillofac Surg. 2011;49:469–473. - PubMed
-
- Alves Cardoso D, Jansen JA, Leeuwenburgh SC. Synthesis and application of nanostructured calcium phosphate ceramics for bone regeneration. J Biomed Mater Res B Appl Biomater. 2012;100:2316–2326. - PubMed
-
- Bauer TW. Bone graft substitutes. Skeletal Radiol. 2007;36:1105–1107. - PubMed
-
- Brandt J, Henning S, Michler G, Hein W, Bernstein A, Schulz M. Nanocrystalline hydroxyapatite for bone repair: an animal study. J Mater Sci Mater Med. 2010;21:283–294. - PubMed
-
- Calasans-Maia M, Calasans-Maia J, Santos S, Mavropoulos E, Farina M, Lima I, et al. Short-term in vivo evaluation of zinc-containing calcium phosphate using a normalized procedure. Mater Sci Eng C Mater Biol Appl. 2014;41:309–319. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

