Mg(2+) differentially regulates two modes of mitochondrial Ca(2+) uptake in isolated cardiac mitochondria: implications for mitochondrial Ca(2+) sequestration
- PMID: 26815005
- PMCID: PMC5098337
- DOI: 10.1007/s10863-016-9644-1
Mg(2+) differentially regulates two modes of mitochondrial Ca(2+) uptake in isolated cardiac mitochondria: implications for mitochondrial Ca(2+) sequestration
Abstract
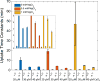
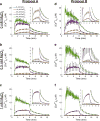
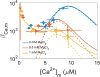
The manner in which mitochondria take up and store Ca(2+) remains highly debated. Recent experimental and computational evidence has suggested the presence of at least two modes of Ca(2+) uptake and a complex Ca(2+) sequestration mechanism in mitochondria. But how Mg(2+) regulates these different modes of Ca(2+) uptake as well as mitochondrial Ca(2+) sequestration is not known. In this study, we investigated two different ways by which mitochondria take up and sequester Ca(2+) by using two different protocols. Isolated guinea pig cardiac mitochondria were exposed to varying concentrations of CaCl2 in the presence or absence of MgCl2. In the first protocol, A, CaCl2 was added to the respiration buffer containing isolated mitochondria, whereas in the second protocol, B, mitochondria were added to the respiration buffer with CaCl2 already present. Protocol A resulted first in a fast transitory uptake followed by a slow gradual uptake. In contrast, protocol B only revealed a slow and gradual Ca(2+) uptake, which was approximately 40 % of the slow uptake rate observed in protocol A. These two types of Ca(2+) uptake modes were differentially modulated by extra-matrix Mg(2+). That is, Mg(2+) markedly inhibited the slow mode of Ca(2+) uptake in both protocols in a concentration-dependent manner, but not the fast mode of uptake exhibited in protocol A. Mg(2+) also inhibited Na(+)-dependent Ca(2+) extrusion. The general Ca(2+) binding properties of the mitochondrial Ca(2+) sequestration system were reaffirmed and shown to be independent of the mode of Ca(2+) uptake, i.e. through the fast or slow mode of uptake. In addition, extra-matrix Mg(2+) hindered Ca(2+) sequestration. Our results indicate that mitochondria exhibit different modes of Ca(2+) uptake depending on the nature of exposure to extra-matrix Ca(2+), which are differentially sensitive to Mg(2+). The implications of these findings in cardiomyocytes are discussed.
Keywords: Calcium efflux; Calcium sequestration; Calcium uptake; Cardiac; Mitochondria.
Figures




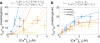
References
-
- An J, Varadarajan SG, Camara A, Chen Q, Novalija E, Gross GJ, et al. Blocking Na(+)/H(+) exchange reduces [Na(+)](i) and [Ca(2+)](i) load after ischemia and improves function in intact hearts. Am J Physiol Heart Circ Physiol. 2001a;281(6):H2398–H2409. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

