Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes
- PMID: 26815411
- PMCID: PMC4963240
- DOI: 10.1002/art.39608
Critical Role of Glucose Metabolism in Rheumatoid Arthritis Fibroblast-like Synoviocytes
Abstract
Objective: Up-regulation of glucose metabolism has been implicated not only in tumor cell growth but also in immune cells upon activation. However, little is known about the metabolite profile in rheumatoid arthritis (RA), particularly in fibroblast-like synoviocytes (FLS). This study was undertaken to evaluate whether changes in glucose metabolism in RA FLS could play a role in inflammation and joint damage.
Methods: Synovium and FLS were obtained from patients with RA and patients with osteoarthritis (OA). The rate of glycolysis after stimulation of FLS with lipopolysaccharide and platelet-derived growth factor BB was measured using glycolysis stress test technology. FLS function was evaluated using a glycolysis inhibitor, 2-deoxy-d-glucose (2-DG). After stimulation of the FLS, a migration scratch assay, MTT assay, and enzyme-linked immunosorbent assay were performed to measure the effect of 2-DG on FLS migration, viability of the FLS, and cytokine secretion, respectively. IRDye 800CW 2-DG was used to assess glucose uptake in the arthritic joints and stromal cells of mice after K/BxN mouse serum transfer. The mice were injected daily, intraperitoneally, with 3-bromopyruvate (BrPa; 5 mg/kg) to assess the effect of inhibition of glycolysis in vivo.
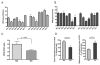
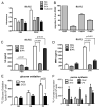
Results: Compared to human OA FLS, the balance between glycolysis and oxidative phosphorylation was shifted toward glycolysis in RA FLS. Glucose transporter 1 (GLUT1) messenger RNA (mRNA) expression correlated with baseline functions of the RA FLS. Glucose deprivation or incubation of the FLS with glycolytic inhibitors impaired cytokine secretion and decreased the rate of proliferation and migration of the cells. In a mouse model of inflammatory arthritis, GLUT1 mRNA expression in the synovial lining cells was observed, and increased levels of glucose uptake and glycolytic gene expression were detected in the stromal compartment of the arthritic mouse joints. Inhibition of glycolysis by BrPa, administered in vivo, significantly decreased the severity of arthritis in this mouse model.
Conclusion: Targeting metabolic pathways is a novel approach to understanding the mechanisms of disease. Inhibition of glycolysis may directly modulate synoviocyte-mediated inflammatory functions and could be an effective treatment strategy for arthritis.
© 2016, American College of Rheumatology.
Figures
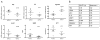



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

