Reversal of MicroRNA Dysregulation in an Animal Model of Pulmonary Hypertension
- PMID: 26815432
- PMCID: PMC4731388
- DOI: 10.1371/journal.pone.0147827
Reversal of MicroRNA Dysregulation in an Animal Model of Pulmonary Hypertension
Abstract
Background: Animals models have played an important role in enhancing our understanding of the pathogenesis of pulmonary arterial hypertension (PAH). Dysregulation of the profile of microRNAs (miRNAs) has been demonstrated in human tissues from PAH patients and in animal models. In this study, we measured miRNA levels in the monocrotaline (MCT) rat model of PAH and examined whether blocking a specific dysregulated miRNA not previously reported in this model, attenuated PAH. We also evaluated changes in miRNA expression in lung specimens from MCT PAH rats overexpressing human prostacyclin synthase, which has been shown to attenuate MCT PAH.
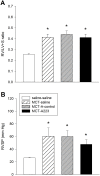
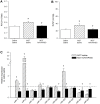
Methods: Expression levels of a panel of miRNAs were measured in MCT-PAH rats as compared to naïve (saline) control rats. Subsequently, MCT PAH rats were injected with a specific inhibitor (antagomiR) for miR-223 (A223) or a nonspecific control oligonucleotide (A-control) 4 days after MCT administration, then weekly. Three weeks later, RV systolic pressure and RV mass were measured. Total RNA, isolated from the lungs, microdissected pulmonary arteries, and right ventricle, was reverse transcribed and real-time quantitative PCR was performed. MiRNA levels were also measured in RNA isolated from paraffin sections of MCT-PAH rats overexpressing prostacyclin synthase.
Results: MiRs 17, 21, and 223 were consistently upregulated, whereas miRs 126, 145, 150, 204, 424, and 503 were downregulated in MCT PAH as compared to vehicle control. A223 significantly reduced levels of miR-223 in PA and lungs of MCT PAH rats as compared to levels measured in A-control or control MCT PAH rats, but A223 did not attenuate MCT PAH. Right ventricular mass and right ventricular systolic pressure in rats treated with A223 were not different from values in A-control or MCT PAH rats. In contrast, analysis of total RNA from lung specimens of MCT PAH rats overexpressing human prostacyclin synthase (hPGIS) demonstrated reversal of MCT-induced upregulation of miRs 17, 21, and 223 and an increase in levels of miR-424 and miR-503. Reduction in bone morphogenetic receptor 2 (BMPR2) messenger (m)RNA expression was not altered by A223, whereas human prostacyclin synthase overexpression restored BMPR2 mRNA to levels in MCT PAH to levels measured in naive controls.
Conclusions: Inhibition of miR-223 did not attenuate MCT PAH, whereas human prostacyclin synthase overexpression restored miRNA levels in MCT PAH to levels detected in naïve rats. These data may establish a paradigm linking attenuation of PAH to restoration of BMPR2 signaling.
Conflict of interest statement
Figures




Similar articles
-
Attenuation of monocrotaline-induced pulmonary hypertension by luminal adeno-associated virus serotype 9 gene transfer of prostacyclin synthase.Hum Gene Ther. 2014 Jun;25(6):498-505. doi: 10.1089/hum.2013.187. Epub 2014 Mar 26. Hum Gene Ther. 2014. PMID: 24512101 Free PMC article.
-
Endothelial-like progenitor cells engineered to produce prostacyclin rescue monocrotaline-induced pulmonary arterial hypertension and provide right ventricle benefits.Circulation. 2013 Aug 27;128(9):982-94. doi: 10.1161/CIRCULATIONAHA.113.003139. Epub 2013 Jul 10. Circulation. 2013. PMID: 23841984
-
Repeated gene transfer of naked prostacyclin synthase plasmid into skeletal muscles attenuates monocrotaline-induced pulmonary hypertension and prolongs survival in rats.Hum Gene Ther. 2004 Dec;15(12):1270-8. doi: 10.1089/hum.2004.15.1270. Hum Gene Ther. 2004. PMID: 15684702
-
Exploring the monocrotaline animal model for the study of pulmonary arterial hypertension: A network approach.Pulm Pharmacol Ther. 2015 Dec;35:8-16. doi: 10.1016/j.pupt.2015.09.007. Epub 2015 Sep 21. Pulm Pharmacol Ther. 2015. PMID: 26403584 Review.
-
microRNA and Pulmonary Hypertension.Adv Exp Med Biol. 2015;888:237-52. doi: 10.1007/978-3-319-22671-2_12. Adv Exp Med Biol. 2015. PMID: 26663186 Review.
Cited by
-
Epigenetic Targets for Oligonucleotide Therapies of Pulmonary Arterial Hypertension.Int J Mol Sci. 2020 Dec 3;21(23):9222. doi: 10.3390/ijms21239222. Int J Mol Sci. 2020. PMID: 33287230 Free PMC article. Review.
-
miR-150-PTPMT1-cardiolipin signaling in pulmonary arterial hypertension.Mol Ther Nucleic Acids. 2020 Nov 4;23:142-153. doi: 10.1016/j.omtn.2020.10.042. eCollection 2021 Mar 5. Mol Ther Nucleic Acids. 2020. PMID: 33335799 Free PMC article.
-
LncRNA-536 and RNA-Binding Protein RBM25 Interactions in Pulmonary Artery Smooth Muscle Cells: Implications in Pulmonary Hypertension.Arterioscler Thromb Vasc Biol. 2025 Aug;45(8):e374-e391. doi: 10.1161/ATVBAHA.125.322734. Epub 2025 Jun 26. Arterioscler Thromb Vasc Biol. 2025. PMID: 40567228
-
Perillyle alcohol and Quercetin ameliorate monocrotaline-induced pulmonary artery hypertension in rats through PARP1-mediated miR-204 down-regulation and its downstream pathway.BMC Complement Med Ther. 2020 Jul 13;20(1):218. doi: 10.1186/s12906-020-03015-1. BMC Complement Med Ther. 2020. PMID: 32660602 Free PMC article.
-
Role of miR-223-3p in pulmonary arterial hypertension via targeting ITGB3 in the ECM pathway.Cell Prolif. 2019 Mar;52(2):e12550. doi: 10.1111/cpr.12550. Epub 2018 Dec 3. Cell Prolif. 2019. PMID: 30507047 Free PMC article.
References
-
- McLaughlin VV, Shillington A, Rich S. Survival in primary pulmonary hypertension: the impact of epoprostenol therapy. Circulation. 2002;106(12):1477–82. . - PubMed
-
- Kang K, Peng X, Zhang X, Wang Y, Zhang L, Gao L, et al. MicroRNA-124 Suppresses the Transactivation of Nuclear Factor of Activated T Cells by Targeting Multiple Genes and Inhibits the Proliferation of Pulmonary Artery Smooth Muscle Cells. The Journal of biological chemistry. 2013;288(35):25414–27. 10.1074/jbc.M113.460287 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

