The 78-kD Glucose-Regulated Protein Regulates Endoplasmic Reticulum Homeostasis and Distal Epithelial Cell Survival during Lung Development
- PMID: 26816051
- PMCID: PMC4942208
- DOI: 10.1165/rcmb.2015-0327OC
The 78-kD Glucose-Regulated Protein Regulates Endoplasmic Reticulum Homeostasis and Distal Epithelial Cell Survival during Lung Development
Abstract
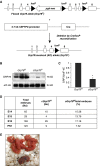
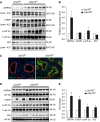
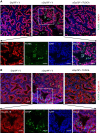
Bronchopulmonary dysplasia (BPD), a chronic lung disease of prematurity, has been linked to endoplasmic reticulum (ER) stress. To investigate a causal role for ER stress in BPD pathogenesis, we generated conditional knockout (KO) mice (cGrp78(f/f)) with lung epithelial cell-specific KO of Grp78, a gene encoding the ER chaperone 78-kD glucose-regulated protein (GRP78), a master regulator of ER homeostasis and the unfolded protein response (UPR). Lung epithelial-specific Grp78 KO disrupted lung morphogenesis, causing developmental arrest, increased alveolar epithelial type II cell apoptosis, and decreased surfactant protein and type I cell marker expression in perinatal lungs. cGrp78(f/f) pups died immediately after birth, likely owing to respiratory distress. Importantly, Grp78 KO triggered UPR activation with marked induction of the proapoptotic transcription factor CCAAT/enhancer-binding proteins (C/EBP) homologous protein (CHOP). Increased expression of genes involved in oxidative stress and cell death and decreased expression of genes encoding antioxidant enzymes suggest a role for oxidative stress in alveolar epithelial cell (AEC) apoptosis. Increased Smad3 phosphorylation and expression of transforming growth factor-β/Smad3 targets Cdkn1a (encoding p21) and Gadd45a suggest that interactions among the apoptotic arm of the UPR, oxidative stress, and transforming growth factor-β/Smad signaling pathways contribute to Grp78 KO-induced AEC apoptosis and developmental arrest. Chemical chaperone Tauroursodeoxycholic acid reduced UPR activation and apoptosis in cGrp78(f/f) lungs cultured ex vivo, confirming a role for ER stress in observed AEC abnormalities. These results demonstrate a key role for GRP78 in AEC survival and gene expression during lung development through modulation of ER stress, and suggest the UPR as a potential therapeutic target in BPD.
Keywords: 78-kD glucose-regulated protein knockout; alveolar epithelial cells; apoptosis; endoplasmic reticulum stress/unfolded protein response signaling.
Figures






Similar articles
-
Grp78 Loss in Epithelial Progenitors Reveals an Age-linked Role for Endoplasmic Reticulum Stress in Pulmonary Fibrosis.Am J Respir Crit Care Med. 2020 Jan 15;201(2):198-211. doi: 10.1164/rccm.201902-0451OC. Am J Respir Crit Care Med. 2020. PMID: 31738079 Free PMC article.
-
Different Roles of GRP78 on Cell Proliferation and Apoptosis in Cartilage Development.Int J Mol Sci. 2015 Sep 7;16(9):21153-76. doi: 10.3390/ijms160921153. Int J Mol Sci. 2015. PMID: 26370957 Free PMC article.
-
Asbestos-induced alveolar epithelial cell apoptosis. The role of endoplasmic reticulum stress response.Am J Respir Cell Mol Biol. 2013 Dec;49(6):892-901. doi: 10.1165/rcmb.2013-0053OC. Am J Respir Cell Mol Biol. 2013. PMID: 23885834 Free PMC article.
-
Systemic effects of AGEs in ER stress induction in vivo.Glycoconj J. 2016 Aug;33(4):537-44. doi: 10.1007/s10719-016-9680-4. Epub 2016 May 28. Glycoconj J. 2016. PMID: 27236787 Review.
-
Endoplasmic Reticulum Stress and Cancer: Could Unfolded Protein Response Be a Druggable Target for Cancer Therapy?Int J Mol Sci. 2023 Jan 13;24(2):1566. doi: 10.3390/ijms24021566. Int J Mol Sci. 2023. PMID: 36675080 Free PMC article. Review.
Cited by
-
Mesencephalic astrocyte-derived neurotropic factor is an important factor in chondrocyte ER homeostasis.Cell Stress Chaperones. 2019 Jan;24(1):159-173. doi: 10.1007/s12192-018-0953-7. Epub 2018 Dec 12. Cell Stress Chaperones. 2019. PMID: 30543055 Free PMC article.
-
Sevoflurane relieves hepatic ischemia-reperfusion injury by inhibiting the expression of Grp78.Biosci Rep. 2018 Oct 5;38(5):BSR20180549. doi: 10.1042/BSR20180549. Print 2018 Oct 31. Biosci Rep. 2018. PMID: 30217942 Free PMC article.
-
Unraveling the Impact of Dab1 Gene Silencing on the Expression of Autophagy Markers in Lung Development.Life (Basel). 2024 Feb 28;14(3):316. doi: 10.3390/life14030316. Life (Basel). 2024. PMID: 38541642 Free PMC article.
-
Endoplasmic Reticulum Stress in Bronchopulmonary Dysplasia: Contributor or Consequence?Cells. 2024 Oct 26;13(21):1774. doi: 10.3390/cells13211774. Cells. 2024. PMID: 39513884 Free PMC article. Review.
-
Secretion of the endoplasmic reticulum stress protein, GRP78, into the BALF is increased in cigarette smokers.Respir Res. 2017 May 2;18(1):78. doi: 10.1186/s12931-017-0561-6. Respir Res. 2017. PMID: 28464871 Free PMC article.
References
-
- Ron D, Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat Rev Mol Cell Biol. 2007;8:519–529. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

