Organic chemistry. Strain-release amination
- PMID: 26816372
- PMCID: PMC4730898
- DOI: 10.1126/science.aad6252
Organic chemistry. Strain-release amination
Abstract
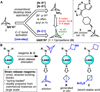
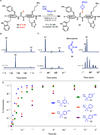
To optimize drug candidates, modern medicinal chemists are increasingly turning to an unconventional structural motif: small, strained ring systems. However, the difficulty of introducing substituents such as bicyclo[1.1.1]pentanes, azetidines, or cyclobutanes often outweighs the challenge of synthesizing the parent scaffold itself. Thus, there is an urgent need for general methods to rapidly and directly append such groups onto core scaffolds. Here we report a general strategy to harness the embedded potential energy of effectively spring-loaded C-C and C-N bonds with the most oft-encountered nucleophiles in pharmaceutical chemistry, amines. Strain-release amination can diversify a range of substrates with a multitude of desirable bioisosteres at both the early and late stages of a synthesis. The technique has also been applied to peptide labeling and bioconjugation.
Copyright © 2016, American Association for the Advancement of Science.
Figures



Comment in
-
Click chemistry: Straining to react.Nat Chem. 2016 Apr;8(4):296-7. doi: 10.1038/nchem.2485. Nat Chem. 2016. PMID: 27001724 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

